The equilibrium constant (K_{mathrm{a}}) for the reaction is (6.0 times 10^{-3}). a. Calculate the (mathrm{pH}) of a
Question:
The equilibrium constant \(K_{\mathrm{a}}\) for the reaction
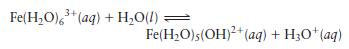
is \(6.0 \times 10^{-3}\).
a. Calculate the \(\mathrm{pH}\) of a \(0.10 \mathrm{M}\) solution of \(\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}ight)_{6}{ }^{3+}\).
b. Calculate the \(\mathrm{pH}\) necessary for \(99.90 \%\) of the iron(III) to be in the form \(\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}ight)_{6}{ }^{3+}\).
c. Will a \(1.0 \mathrm{M}\) solution of iron(II) nitrate have a higher or lower \(\mathrm{pH}\) than a \(1.0 \mathrm{M}\) solution of iron(III) nitrate? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





