The following are representations of acidbase reactions: + 11 + +
Question:
The following are representations of acid–base reactions: 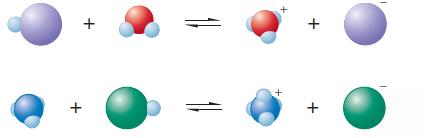
Transcribed Image Text:
+ 11 呢 + +
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
HCl H2O H3...View the full answer

Answered By

Godswill Okorie
M.Sc chemistry specialization in organic chemistry, B.ed .I am having industry experience of seven years working with Ranbaxy nd Shimadzu analytical India by working as an application chemistry.I am having good practical experience on chromatography techniques,which later helped me in my teaching.I worked as PGT chemistry teacher with KV and APS.
As a teacher I was able to achieve good results with my students.I used to take 11th and 12th chemistry and science to classes 7th ,8th and ninth. While teaching I used to guide students for various carrier opportunities.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following are representations of acid base reactions: a. Label each of the species in both equations as an acid or a base and explain. b. For those species that are acids, which labels apply:...
-
The following are representations of two forms of glucose. The six-membered ring is known to exist in a chair conformation in each form. Draw clear representations of the most stable conformation of...
-
The following are representations of two forms of glucose. The six-membered ring is known to exist in a chair conformation in each form. Draw clear representations of the most stable conformation of...
-
M1 is a way to measure... a) the level of bank reserves b) a country's money supply c) the level of savings in a country d) a country's economic potential
-
Suppose that X and Y are independent random variables, that X has the uniform distribution on the integers 1, 2, 3, 4, 5 (discrete), and that Y has the uniform distribution on the interval [0, 5]...
-
Chelidonic acid, a 4-oxacyclohexanone (common name, g-pyrone), is found in a number of plants and is synthesized from acetone and diethyl ethanedioate. Formulate a mechanism for this transformation.
-
Why are some deductions called above the line deductions and others called below the line deductions? What is the line?
-
The National Occupant Protection Use Survey (NOPUS) was conducted to provide probability-based data on motorcycle helmet use in the United States. The survey was conducted by sending observers to...
-
Class work Tom Key, Tom Carr and Tom King are partners of a firm known as Tom & Tom. Their Partnership Agreement stipulated that: Profit and loss are to be shared equally ii Tom King is entitled to...
-
Suppose that the evolving star is the same size as our Sun at the start of the animation. The white dwarf, meanwhile, is only about as large as the Eartheven though it may have two-thirds or...
-
You have two solutions of the salts NaX(aq) and NaY(aq) at equal concentrations. What would you need to know to determine which solution has the higher pH? Explain how you would decide (perhaps even...
-
Acids and bases can be thought of as chemical opposites (acids are proton donors, and bases are proton acceptors). Therefore, one might think that K a = 1/K b . Why isnt this the case? What is the...
-
If you presided over an international organization and wanted to increase the population doubling time in Bangladesh, what specific policy would you suggest? Explain your choice.
-
Briefly, discuss the use of survey research in exploratory, descriptive, explanatory, and evaluation studies. Using a criminal justice example select one type of research study and develop one...
-
Medical Helicopters In a study of helicopter usage and patient survival, results were obtained from 47,637 patients transported by helicopter and 111,874 patients transported by ground (based on data...
-
Woodland Hills Company reported income before taxes (pretax financial income) in its income statement of $60,000. Among the items included in the computation of pretax financial income were the...
-
cest Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this...
-
The activation energy for the gas phase decomposition of isobutyl bromide is 211 kJ. (CH3)2CHCH2 Br (CH3)2C=CH2+ HBr The rate constant at 676 K is 5.73 x 10-4 s. The rate constant will be 0.00647 s...
-
Rank these planets in order from longest to shortest year: (a) Mercury, (b) Venus, (c) Earth.
-
Write a paper about how diet relates to breast cancer in women study design to use: case control study purpose & rationale the purpose of this final project is to utilize the methods and...
-
A mixture of chromium and zinc weighing 0.362 g was reacted with an excess of hydrochloric acid. After all the metals in the mixture reacted, 225 mL of dry hydrogen gas was collected at 27 o C and...
-
You have a sealed, flexible balloon filled with argon gas. The atmospheric pressure is 1.00 atm and the temperature is 25oC. The air has a mole fraction of nitrogen of 0.79, the rest being oxygen. a....
-
Derive a linear relationship between gas density and tem-perature, and use it to estimate the value of absolute zero temperature (in o C to the nearest 0.1 o C) from an air sample whose density is...
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


