The following flasks show the dissociation of a diatomic molecule, X 2 , over time. (a) Which
Question:
The following flasks show the dissociation of a diatomic molecule, X2, over time.
(a) Which flask represents the point in time at which the reaction has reached equilibrium?
(b) What percentage of the X2 molecules has decomposed at equilibrium?
(c) Assuming that the initial pressure of X2 was 0.10 bar, calculate the value of K for the decomposition.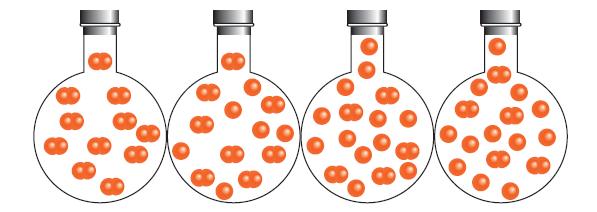
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Flask 3 rep...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The molar heat capacity of a diatomic molecule is 29.1 J/K mol. Assuming the atmosphere contains only nitrogen gas and there is no heat loss, calculate the total heat intake (in kilojoules) if the...
-
Assume that on January 1, Year 2, several supporters of the entity spend their own time and money to construct a garage for the entity's vehicles. The results are donated for free. The labor has a...
-
In Problems 23 34, graph each system of linear inequalities. x - 2y 6 2x - 4y 0
-
When a mixture of ethanol and water is distilled at ambient pressure, the products are a distillate of ethanol and water of nearazeotrope composition (89.4 mol% ethanol) and a bottoms product of...
-
Two shafts 20 ft apart, with axes in the same horizontal plane, are to be connected with a flat belt in which the driving pulley, powered by a six-pole squirrel-cage induction motor with a 100 brake...
-
(Analysis of Given Ratios) Picasso Company is a wholesale distributor of professional equipment and supplies. The companys sales have averaged about $900,000 annually for the 3-year period 20032005....
-
On January 1, 2012, Jungle Company sold a machine to Safari Company for $30,000. The machine had an original cost of $24,000, and accumulated depreciation on the asset was $9,000 at the time of the...
-
Using the information below, compute the days' sales in raw materials inventory: Raw materials used$ 121,600Beginning raw materials inventory18,000Ending raw materials inventory20,200 Select one: a....
-
A double pipe heat exchanger is made of a 6-nom sch 40 commercial steel outer pipe and a 5-nom sch 40S stainless steel inner pipe. The fluid in the annular space is cyclohexane that has a volumetric...
-
Use the phase diagram for helium in Exercise 5B.3 (a) To describe the phases in equilibrium at each of heliums two triple points; (b) To decide which liquid phase is the more dense, helium-I or...
-
The vapor pressure of benzene, C 6 H 6 , is 94.6 Torr at 25 8C. A nonvolatile compound was added to 0.300 mol C 6 H 6 (l) at 25C and the vapor pressure of the benzene in the solution decreased to...
-
The famous Fibonacci sequence was proposed by Leonardo Pisano, also known as Fibonacci, in about a.d. 1200 as a model for the growth of rabbit populations. It is given by the recurrence relation f n...
-
Use least square regression to fit a straight line to the following data taken from the conductance (S/m) of a material with respect to temperature (C) of a composite material used to absorb heat....
-
A pile group consists of nine friction piles in clay soil (see Figure 10-40). The diameter of each pile is 16 in., and the embedded length is 30 ft each. Center-to-center pile spacing is 4 ft. Soil...
-
The rigid bar EBC is supported by two links AB and CD as shown in Figure 1. The Link AB is made of aluminum (E = 70 GPa) and the link CD is made of steel (E = 200 GPa). Both links have a Width = 30...
-
a well-insulated storage tank was pressurized under ideal gas conditions by air flowing into the tank. We used the first law to estimate the final temperature of the gas in the tank, Tf,tank- = We...
-
Transportation of natural gas is commonly done via pipelinesacross long distances. A company uses a 0.6-m diameter pipe totransport natural gas. Then pumping stations are located atdifferent points...
-
You are advising a small company. a) Would you recommend using a firewall? Explain. b) Would you recommend using antivirus filtering? Explain. c) Would you recommend an intrusion detection system?...
-
1. Firms may hold financial assets to earn returns. How the firm would classify financial assets? What treatment will such financial assets get in the financial statements in accordance with US GAAP...
-
A 2.25 mole sample of an ideal gas with C V ,m = 3/2R initially at 310. K and 1.25 10 5 Pa undergoes a reversible adiabatic compression. At the end of the process, the pressure is 3.10 10 6 Pa....
-
Propose a structure for a compound with molecular formula C 3 H 8 O that exhibits the following 1 H NMR and 13C NMR spectra: Proton NMR 0.5 5.0 4.5 4.0 3.5 3.0 2.0 1.5 1.0 25 Chemical shift (ppm)...
-
Propose two possible structures for a compound with molecular formula C 5 H 12 O that exhibits the following 13 C NMR and IR spectra: Carton 13 NMR 29.1 9.5- 73.8- 100 90 80 70 60 50 20 10 Chemical...
-
Vaughn Company sells two types of pumps. One is large and is for commercial use. The other is smaller and is used in residential swimming pools. The following inventory data is available for the...
-
To fund your dream around-the-world vacation, you plan to save $1,300 per year for the next 14 years starting one year from now. If you can earn an interest rate of 5.83 percent, how much will you...
-
On NSE (Indian stock exchange), shares of ICICI Bank trade for 935 rupees. If the spot exchange rate is USD 0.012, what is the no-arbitrage USD price of ICICI Bank ADR? Assume that transactions costs...

Study smarter with the SolutionInn App


