The following Lewis structure was drawn for a Period 3 element. Identify the element. CI :0: E-
Question:
The following Lewis structure was drawn for a Period 3 element. Identify the element.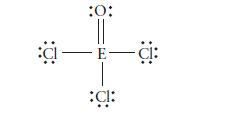
Transcribed Image Text:
CI :0: E- :15: - CI:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
E i...View the full answer

Answered By

Raunak Agarwal
Teaching is my hobby and now my profession. I teach students of CA and CFA(USA) in batches of 100 students and have a 5 year experience.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The following Lewis structure was drawn for a Period 4 element. Identify the element. :0: E=0 :CI:
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
A second Lewis structure can be drawn for one of the nucleophiles in Problem 36. (a) Identify it and draw its alternate structure (which is simply a second resonance form), (b) Is there a second...
-
Many auditors consider the substantiation of the figure for inventory to be a more difficult and challenging task than the verification of most other items on the balance sheet. List several specific...
-
Prepare the journal entries that the lessee should make to record the following transactions. 1. The lessee makes a lease payment of $80,000 to the less or in an operating lease transaction. 2....
-
The comparative balance sheet of Hirayama Industries Inc. for December 31, 20Y2 and 20Y1, is as follows: The following additional information is taken from the records: 1. Land was sold for $153. 2....
-
What is job-order costing?
-
The section of the sealed joint shown in the figure is loaded by a repeated force P = 6 kip. The members have E = 16 Mpsi. All bolts have been carefully preloaded to Fi = 25 kip each
-
When preparing for a business trip to China, Kaylee Putbrese determined she needed to bring $5,200. How much must she borrow for a simple discount note at 6% for 45 days? (Use 360 days a year. Round...
-
Identify the hybrid orbitals used by the atom in boldface red type in each of the following species: (a) BF 3 ; (b) AsF 3 ; (c) BrF 3 ; (d) SeF 3 + .
-
State the hybridization of the atom in boldface red type in each of the following molecules and ions: (a) SF 6 ; (b) ClO 3 ; (c) NO 3 ; (d) OCCl2 .
-
Let s be the mean salary (in dollars) for people with t years of education.
-
A 447 gram cart (mA) slides along a very smooth track and collides with a stationary 475 gram cart (mB). A motion detector records the velocity of cart A, as shown in Figures 1 and 2. A force probe...
-
M8 Homework i Saved 1 Mayfair Company completed the following transactions and uses a perpetual inventory system. Help Save & Exit Submit Check my work 10 points eBook Print References June 4 Sold...
-
Free Response Table Problem x -6 -80 -4 -3 f(x) 1.948 1 0 -2 -2.005 -798 undefined -2 -1.995 0 1 1.995 2 2.005 6 80 802 4 3.333 3.001 undefined 2.998 2.5 2.048 23. The table above represents values...
-
5. [-/0 Points] DETAILS OSPRECALC1 2.2.106. Use algebra to find the point at which the line f(x) = -x 258 -X+ intersects the line h(x) = x+ 91 + 25 10 (x, y) = Additional Materiale MY N
-
What does the graph tells? from your own understanding. CoursHeroTranscribedText 136 DIVIDED ATTENTION COUNTED TIME BACKWARDS 134 1 2 3 130 136 UNDIVIDED ATTENTION COUNTED TIME BACKWARDS 134 5 132...
-
Nelsonville Inc. budgeted factory overhead at $255,000 for the period for Department A, based on a budgeted volume of 50,000 machine hours. At the end of the period, the actual factory overhead was...
-
Cornell and Roberts are partners who agree to admit Stanley to their partnership. Cornell has a capital balance of $80,000 and Roberts has a capital balance of $120,000. Cornell and Roberts share net...
-
A solution contains 1.0 3 1026 M HOCl and an unknown concentration of KOCl. If the pH of the solution is 7.20, calculate the KOCl concentration.
-
A solution contains 1.0 3 1026 M HOCl and an unknown concentration of KOCl. If the pH of the solution is 7.20, calculate the KOCl concentration.
-
An aqueous solution contains dissolved C 6 H 5 NH 3 Cl and C 6 H 5 NH 2 . The concentration of C 6 H 5 NH 2 is 0.50 M and pH is 4.20. a. Calculate the concentration of C 6 H 5 NH 3 + in this buffered...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


