The following peaks were observed in the photoelectron spectra for two elements (see Exercise 1.11). Identify the
Question:
The following peaks were observed in the photoelectron spectra for two elements (see Exercise 1.11). Identify the elements, write their electron configurations, and explain your reasoning: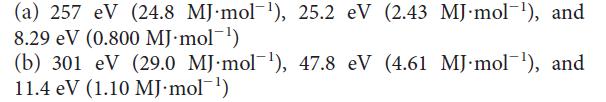
Exercise 1.11
Photoelectron spectroscopy can be used to determine the energies of atomic orbitals by measuring the energies required to remove electrons from them. The following peaks were observed in the photoelectron spectra for two elements.
Identify the elements, write their electron configurations, and explain your reasoning:
75.7 eV (7.30 MJ· mol-1) and 5.38 eV (0.519 MJ · mol-1)
153 eV (14.8 MJ· mol-1) and 9.33 eV (0.90 MJ· mol-1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





