The following plot shows the distribution of speeds for N 2 at 300, 500, and 1000 K.
Question:
The following plot shows the distribution of speeds for N2 at 300, 500, and 1000 K.
(a) Identify the temperature of the gas for each curve.
(b) What is the root mean square speed of N2 molecules at 227°C?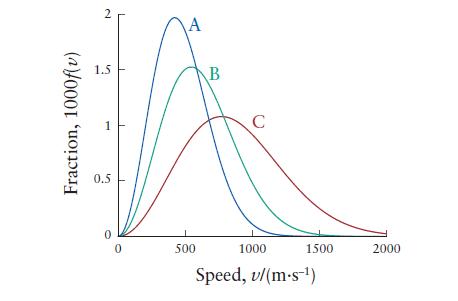
Transcribed Image Text:
Fraction, 1000f(v) 2 1.5 0.5 0 0 A 500 B C 1000 1500 Speed, v/(m.s-¹) 2000
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a A 300 ...View the full answer

Answered By

Mercy Kangai
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past seven years. I can bring value to your business and help solve your administrative assistant issues. I have extensive experience in marketing and small business management.
4.80+
27+ Reviews
86+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
A particle of mass 167 g is attached to a horizontal spring and set into oscillatory motion on a frictionless surface by stretching the spring by 67 cm from its equilibrium length at t=0.00 s. The...
-
The following figure shows the distribution of speeds for two samples of N 2 gas. One sample is at 300 K, and the other is at 1000k. Which is which? N()/Ntotal 0 500 1000 1500 Molecular speed (m/s)...
-
Del Gato Clinic's cash account shows an $14,180 debit balance and its bank statement shows $13,236 on deposit at the close of business on June 30. a. Outstanding checks as of June 30 total $1,502. b....
-
pick 1 1 point A small island nation has license plates that consist of 2 letters ( \( A-Z \) allowed) followed by 3 numbers (0 \( -9 \) allowed). How many plates are possible that do not repeat the...
-
The towline exerts a force of P = 4 kN at the end of the 20-m-long crane boom. If x = 25 m, determine the position ? of the boom so that this force creates a maximum moment about point O. What is...
-
The following figure is a Normal probability plot of the emissions of carbon dioxide (CO 2 ) per person in 48 countries.15 Use the graph to determine if this distribution of CO 2 emissions is...
-
TelComm Corporation is a manufacturer of components for the cell phone industry. TelComm founder Alex Bell heard that China has the worlds largest number of cell phone users and wants to begin...
-
McDonald Corp. reported the following on its comparative income statement: Prepare a horizontal analysis of revenues and gross profit both in dollar amounts and in percentages for 2017 and 2016. (in...
-
it is not 224,400. please show work. thank you. Through the payment of $15,216,500 in cash, Drexel Company acquires voting control over Young Company. This price is paid for 60 percent of the...
-
One form of silicon has density of 2.33 g cm 3 and crystallizes in a cubic lattice with a unit cell edge of 543 pm. (a) What is the mass of each unit cell? (b) How many silicon atoms does one unit...
-
A sample of methane gas, CH 4 , was slowly heated at a constant pressure of 0.90 bar. The volume of the gas was measured at a series of different temperatures and a plot of volume against temperature...
-
A university conducted a survey of 366 undergraduate students regarding satisfaction with student government. Results of the survey are shown in the table by class rank. (a) If a survey participant...
-
Part 2 One Stop Electrical Shop are merchandisers of household fixtures & fittings. The business began the last quarter of 2020 (October to December) with 25 Starburst Wall Clocks at a total cost of...
-
A species of butterfly has three subspecies A, B, and C. A scientist is trying to classify observed. specimens into these subspecies based on the color of their wings, which can be blue, green, pink,...
-
Stock during the year were sold for $8 per share. On December 31 , Portland had no remaining treasury stock. Required: Prepare the necessary journal entries to record any transactions associated with...
-
2) 20 pts. A 2-kg block rests on a wedge that has a coefficient of friction between the wedge and block of 0.3. The system accelerated to the right. Determine the maximum acceleration of the system...
-
ABC Ltd. is concerning about its poor performance and considering whether or not dropping the production and sells of product R, which incurs losses of Birr 4000. Additional information: The salaries...
-
How many solutions to the equation - 2.6 = 3 sin x occur in the first three positive cycles of the function y = 3 sin x? Explain your answer.
-
Find the image of x = k = const under w = 1/z. Use formulas similar to those in Example 1. y| y = 0 -21 -2 -1 -1, /1 12 T -1 -1 y= -2 x =0
-
Rationalize the following lattice energy values: Lattice Energy (k/mol) -2862 -2130 -2721 -2095 Compound CaSe Na2Se CaTe NazTe
-
The lattice energies of FeCl3, FeCl2, and Fe2O3 are (in no particular order) 2631 kJ/ mol, 5339 kJ/ mol, and 14,774 kJ/ mol. Match the appropriate formula to each lattice energy.
-
Use bond energy values in Table to estimate ÎH for each of the following reactions in the gas phase. a. H2(g) + Cl2(g) 2HCl(g) Table s | 14 39 95 45 72 1 1419 6847064968 77386 42222 34 985 0302...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


