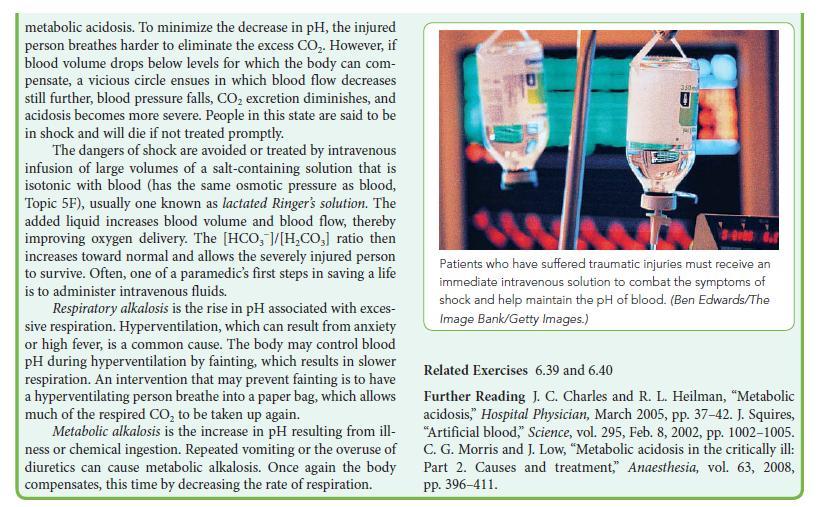
The pH of the blood is maintained by a buffering system consisting primarily of hydrogen carbonate ion
Question:
The pH of the blood is maintained by a buffering system consisting primarily of hydrogen carbonate ion (HCO3–) and H3O+ in equilibrium with water and CO2:![]()
During exercise, CO2 is produced at a rapid rate in muscle tissue.
(a) How does exercise affect the pH of blood?
(b) Hyperventilation (rapid and deep breathing) can occur during intense exertion. How does hyperventilation affect the pH of the blood?
(c) The normal first-aid treatment for hyperventilation is to have the patient breathe into a paper bag. Explain briefly why this treatment works and tell what effect the paper-bag treatment has on the pH of the blood. See Box 6G.1.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





