The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of
Question:
The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of a volatile solute. If the solute is less volatile than water, explain what will happen to the volumes in the two containers as time passes.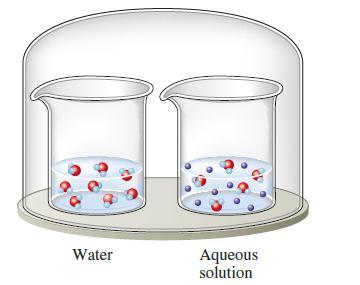
Transcribed Image Text:
Water Aqueous solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
The volume of the aqueous solution will decrease as the solute evaporates The volume o...View the full answer

Answered By

Kenneth Mutia
I have a B.S. in Statistics from the Jomo Kenyatta University of Agriculture and technology. I have been an academic tutor for over 3 years. I have a passion for helping students reach their full potential and am dedicated to helping them succeed. I am patient and adaptable, and I have experience working with students of all ages and abilities, from elementary school to college in their various fields. I have a wide scope of diverse tutoring experience in several courses of study with significant success as a tutor.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of a volatile solute. If the solute is less volatile than water, explain what will happen to the...
-
Explain what will happen to the price level, Real GDP, and the unemployment rate in the following cases: a. AD rises and SRAS is constant. b. AD falls and SRAS is constant. c. AD is constant and SRAS...
-
The practice of charging interest on money that is lent by one party to another, while commonplace now, has been historically controversial. Major religions have prohibited the charging of interest...
-
Write a static method that takes a domain name as its argument and returns the reverse domain name (reverse the order of the strings between periods). For example, the reverse domain name of...
-
The lengths of lumber a machine cuts are normally distributed, with a mean of 96 inches and a standard deviation of 0.5 inch. (a) What is the probability that a randomly selected board cut by the...
-
Lisa is offered a loan from a bank at 4.2% compounded monthly. A credit union offers similar terms, but at a rate of 4.4% compounded semiannually. Which loan should she accept? Present calculations...
-
Whats my line? An eccentric professor believes that a child with IQ 100 should have a reading test score of 50 and predicts that reading score should increase by 1 point for every additional point of...
-
Keller Construction is considering two new investments. Project E calls for the purchase of earthmoving equipment. Project H represents an investment in a hydraulic lift. Keller wishes to use a net...
-
10 pou Using the Transaction Analysis table provided, analyze the effect of the transactions listed below on the extended Accounting Equation. (10 Marks) Nada Al Mansouri started "Zoha, Pharmacy" in...
-
Using the following trial balance and adjustment data for Vivian?s Repair Co. of Windsor, prepare1.?A worksheet for October2. An income statement for October, a statement of owner?s equity for...
-
Vapor-pressure lowering is a colligative property, as are freezing-point depression and boiling-point elevation. What is a colligative property? Why is the freezing point depressed for a solution as...
-
The pressure of CO 2 in a bottle of sparkling wine was calculated assuming that the CO 2 was insoluble in water. This was an incorrect assumption. Redo this problem by assuming that CO 2 obeys Henrys...
-
The number of households currently receiving a daily newspaper has decreased over the last 10 years, and many people state they obtain information about current events through television news and the...
-
9. [10] Suppose that B and W are BMs and that they are correlated with correlation coefficient P (-1, 1) in the sense that the correlation coefficient between Bt and Wt for all t>0. Then we can...
-
You have just incorporated and started your business. Your corporate pre-tax profit is $40,000. This is your only source of income. This income is eligible for the Small Business Deduction and is...
-
4. Provide the information requested in the statements below: a) Find and draw all C's that do not contain H's (if any). For this, redraw the structure where you show the d ('s). N b) Find and draw...
-
Suppose that f(x) = 8x + 5. (A) Find the slope of the line tangent to f(x) at x = 7. (B) Find the instantaneous rate of change of f(x) at x = -7. C) Find the equation of the line tangent to f(x) at x...
-
Whichof the following regarding the relationship between business risk and financial risk is least accurate based on our discussions in class? A. Business risk represents uncertainty caused by...
-
A certain long-distance carrier provides service between Podunk and Nowheresville. If x represents the number of minutes for the call, where x > 0, then the function (x) = 0.40[[x]] + 0.75 gives the...
-
In your audit of Garza Company, you find that a physical inventory on December 31, 2012, showed merchandise with a cost of $441,000 was on hand at that date. You also discover the following items...
-
Predict the molecular structure and the bond angles for each of the following. (See Exercises 89 and 90.) a. XeCl2 b. ICl3 c. TeF4 d. PCl5
-
Predict the molecular structure and the bond angles for each of the following. a. ICl5 b. XeCl4 c. SeCl6
-
Which of the molecules in Exercise 88 have net dipole moments (are polar)? SeO3 SeO2 PCl3 SCl2 SiF4
-
A local bookstore is considering adding a coffee shop to their store. Building the coffee shop will cost $256,340.00 today. The bookstore is going to try this project for five years. The bookstore...
-
What is the depreciation deduction, using each of the following methods, for the second year for an asset that costs $40,000 and has an estimated MV of $5,000 at the its seven-year useful life?...
-
How do we use the simulation analysis for financial decision making

Study smarter with the SolutionInn App


