The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of
Question:
The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of a volatile solute.
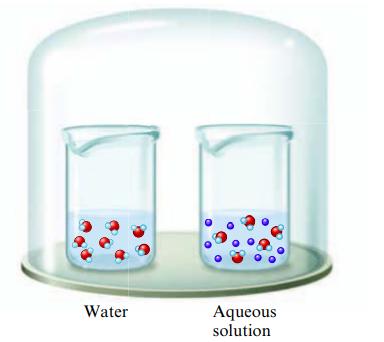
If the solute is less volatile than water, explain what will happen to the volumes in the two containers as time passes.
Transcribed Image Text:
Water Aqueous solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
to answer this question you need to understand that nature attempts to make things equal So if we ha...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of a volatile solute. If the solute is less volatile than water, explain what will happen to the...
-
Explain what will happen to the price level, Real GDP, and the unemployment rate in the following cases: a. AD rises and SRAS is constant. b. AD falls and SRAS is constant. c. AD is constant and SRAS...
-
The practice of charging interest on money that is lent by one party to another, while commonplace now, has been historically controversial. Major religions have prohibited the charging of interest...
-
Consider the integral where n is an integer. Using the trigonometric identity 1 + tan 2 x = sec 2 x, show that and hence obtain the recurrence relation Use this to find (Recurrence relations of this...
-
Describe the security issues firms face in B2B e-commerce. What are some safeguards firms use to reduce their security risks?
-
Arnold exercised an incentive stock option in 2014, acquiring 1,500 shares of stock at an option price of $80 per share. The FMV of the stock at the date of exercise was $110 per share. In 2016, the...
-
35. On January 1, year 1, Jessica received 10,000 shares of restricted stock from her employer, Rocket Corporation. On that date, the stock price was $10 per share. On receiving the restricted stock,...
-
The management of Universal Manufacturing Company (Problem 5-4) likes to have its operators working 90% of the time. What must the assembly line arrival rate be in order for the operators to be as...
-
You are planning to make monthly deposits of $180 into a retirement account that pays 8 percent interest compounded monthly. If your first deposit will be made one month from now, how large will your...
-
1. How does Apple use strategic management to compete? 2. What is leaderships role in Apples strategic implementation? 3. What are key forces in the general and industry environments that affect...
-
What mass of sodium oxalate (Na 2 C 2 O 4 ) is needed to prepare 0.250 L of a 0.100-M solution?
-
If a solution shows positive deviations from Raoults law, would you expect the solution to have a higher or lower boiling point than if it were ideal? Explain.
-
Coca-Cola and Pepsi are competing in the Brazilian soft-drink market. Each firm is deciding whether to follow an aggressive advertising strategy, in which the firm significantly increases its...
-
Turn this information into an excel sheets with the excel formulas being shown P12.2 (LO 1, 2) (Liability Entries and Adjustments) Listed below are selected transactions of Schultz Department Store...
-
1. Consider an undirected random graph on the set of four vertices {A, B, C, D} such that each of the 4 2 = 6 potential edges exists with probability 0.2, independently of the presence/absence of any...
-
Basic Net Present Value Analysis Jonathan Butler, process engineer, knows that the acceptance of a new process design will depend on its economic feasibility. The new process is designed to improve...
-
Determine the support reactions at the smooth collar A and the normal reaction at the roller support B. 800 N 600 N B 0.8 m 0.4 m 0.4 m 0.8 m
-
A plant hopes to cool a steam line by sending it through a throttling valve to expand it to atmospheric pressure. The steam enters the valve at 550C and 250 bar. The expansion in the valve happens so...
-
1. Why didnt HKE ever charge Kelomar the late interest charge until this dispute? 2. Why did the court reject Kelomars argument that it was reasonable to think that HKE had waived the late interest...
-
Wholesalers Ltd. deals in the sale of foodstuffs to retailers. Owing to economic depression, the firm intends to relax its credit policy to boost productivity and sales. The firms current credit...
-
Predict the major product(s) that are expected when each of the following alkenes is treated with Br 2 /H 2 O: a. b. c. d.
-
Muscalure is the sex pheromone of the common housefly and has the molecular formula C 23 H 46 . When treated with O 3 followed by DMS, the following two compounds are produced. Draw two possible...
-
Propose a plausible mechanism for each of the following reactions: a. b. stitl. [H,SO,] Conc. H2SO4
-
Need help filling out these tax forms. Not sure how to do 1040 page 2 or schedule 3. I think I have schedule 1 right but need help with the itemized deductions for 1040 page 1 Required information...
-
Question:What should Airbus and Boing have learned from IBERIA case? What changed in the industry when Boing decided to develop Dreamliner in 2003?( Read the following case and ppt) Airline Route...
-
Which of the following needs to be always assessed when you are evaluating the literature you have obtained for your research? O All of the above O Sufficiency Value O Relevance Several approaches...

Study smarter with the SolutionInn App


