Predict the major product(s) that are expected when each of the following alkenes is treated with Br
Question:
a.
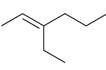
b.
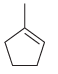
c.
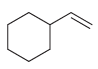
d.
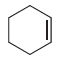
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
a b...View the full answer

Answered By

Morgan Njeri
Very Versatile especially in expressing Ideas in writings.
Passionate on my technical knowledge delivery.
Able to multitask and able to perform under pressure by handling multiple challenges that require time sensitive solution.
Writting articles and video editing.
Revise written materials to meet personal standards and satisfy clients demand.
Help Online Students with their course work.
4.90+
12+ Reviews
38+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the products that are expected when each of the following alkenes is treated with a peroxy acid (such as MCPBA) followed by aqueous acid: a. b. c. d. e. f.
-
Predict the products that are expected when each of the following alkenes is treated with ozone followed by DMS: a. b. c. d. e. f.
-
Draw the major product(s) that are expected when each of the following amines is treated with excess methyl iodide and then heated in the presence of aqueous silver oxide. (a) (b) NH2 NH2
-
What is the formula to find total dividend and payout ratio? This is the information I have: the amount of shares the company holds and the last dividend paid. Lastly, will there be enough cash to...
-
The control environment reflects the company's attitude, awareness, and actions of management and the board concerning the importance of control and how it is used. This is very relevant to an...
-
For each polynomial function, find all zeros and their multiplicities. (x) = (x + 1) (x 1) (x - 10)
-
Suicides among Vietnam veterans. Did the horrors of fighting in Vietnam drive many veterans of that war to suicide? A figure of 150,000 suicides among Vietnam veterans in the 20 years following the...
-
A film of Jesse Owenss famous long jump (Fig.6-42) in the 1936 Olympics shows that his center of mass rose 1.1m from launch point to the top of the are What minimum speed did he need at launch if he...
-
Subject: Management Accounting and Finance Queptien 4 ps linats: Roquiret: wach itherratin. Onaks
-
A quarterback on a football team has a pass completion rate of 0.62. If, in a given game, he attempts 16 passes, what is the probability that he will complete (a) 12 passes? (b) More than half of his...
-
Predict the major product(s) for each of the following reactions: a. b. c. d. Br2 Br2
-
Muscalure is the sex pheromone of the common housefly and has the molecular formula C 23 H 46 . When treated with O 3 followed by DMS, the following two compounds are produced. Draw two possible...
-
Project evaluation and ranking Corliss Company, whose cost of capital is 12 percent, is evaluating two capital projects whose estimated cash savings are as follows: Project A costs $29,200 and...
-
Problem 228: The derivative is dz dt = = atb where a , and b =
-
Write a Python program which will take N names from the user. Create a dictionary from the N names that will hold First_name, Middle_name and Last_name in separate keys. The inputs will take N at...
-
2 Finding Poles and Zeros from a Bode Plot Consider the magnitude portion of the Bode plot in Figure 3. Based on the linear approxi- mation in red, find the transfer function G(s). 5 0 -5 10 -10 -15...
-
Indicate whether the following statements are "TRUE" or "FALSE" 1- Financial accounting is considered to be the backbone to top management. 2- Cost accounting identifies, summarizes and interprets...
-
Refer to case 3 shown above. Assume that Beta Division is now receiving an 3% price discount from the outside supplier. a. What is Alpha Division's lowest acceptable transfer price? b. What is Beta...
-
In Exercises 17 through 22, you are given the price p(q) at which q units of a particular commodity can be sold and the total cost C(q) of producing the q units. In each case: (a) Find the revenue...
-
Identify the Critical Infrastructure Physical Protection System Plan.
-
How can you account for the fact that cis-1, 3-pentadicne is much less reactive than trans-1, 3-pentadiene in the DielsAlder reaction?
-
Would you expect a conjugated diyne such as 1, 3-butadlyne to undergo DielsAlder reaction with a dienophile explain.
-
Reaction of isoprene (2-methyl-1, 3-hutadiene) with ethyl propenoate gives a mixture of two Diels?Alder adducts. Show the structure of each, and explain why a mixture is formed. CO2CH2CH3
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


