The unit cell for a pure xenon fluoride compound is shown below. What is the formula of
Question:
The unit cell for a pure xenon fluoride compound is shown below. What is the formula of the compound? 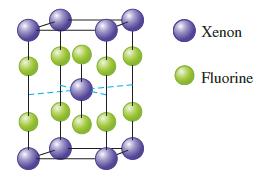
Transcribed Image Text:
Xenon Fluorine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
Answer The formula of the compound is XeF4 Explanation The formula of t...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The unit cell for a pure xenon fluoride compound is shown below. What is the formula of the compound? Xenon Fluorine
-
The unit cell for a pure xenon fluoride compound is shown below. What is the formula of the compound? Xenon Fluorine
-
Changing preferences can also affect changes in land use. In the United States, the proportion of the population in the 65-and-older age bracket is growing. What effects might this have on the...
-
For a sample of n = 64, find the probability of a sample mean being less than 24.3 when = 24 and = 1.25. The population mean and standard deviation are given. Find the indicated probability and...
-
Avivas pay stub shows gross earnings of $731.92 for a week. Her regular rate of pay is $15.80 per hour for a 35-hour week and overtime is paid at time-and-a-half regular pay. How many hours did she...
-
Hot dogs Are hot dogs that are high in calories also high in salt? The figure below is a scatterplot of the calories and salt content (measured as milligrams of sodium) in 17 brands of meat hot...
-
Hitachi, Ltd., reports total revenues of 9,041,071 million for its fiscal year ending March 31, 2013, and its March 31, 2013, unadjusted trial balance reports a debit balance for trade receivables...
-
I have the worksheet above. I need the two charts filled out correctly. Income Statement Debit Credit - Account Number Name 1110 Cash 1120 Accounts Receivable 1130 Prepaid Insurance 1140 Prepaid Rent...
-
The following transactions were completed by Almeda Inc., whose fiscal year is the calendar year: Year 1 July 1. Issued $75,000,000 of 10-year, 9% callable bonds dated July 1, Year 1, at a market...
-
Consider the following melting point data: Compound NaCl MgCl AlCl3 SiCl4 PC13 SCl Cl mp (C) 801 708 190 -70 -91 -78 -101 Compound NaF mp (C) MgF 997 1396 AlF3 1040 -90 SiF4 PF5 SF6 F -94 -56-220
-
Use the diagram of the unit cell for the hexagonal closest packed structure in Fig. 16.14 to determine the net number of atoms in the hcp unit cell. Fig. 16.14 b a b 5 10 hep 12 3 Figure 16.16 The...
-
The following table lists the weekly quantities and routings of ten parts that are being considered for cellular manufacturing in a machine shop. Parts are identified by letters and machines are...
-
1. Using appropriate examples, compare and contrast the genetic diversity of marine fish species with freshwater fish species (8 marks) 2. Your class went on a trip and discovered a crater lake on...
-
Find sin(29) given that cos(0) = and 0
-
Amazing Aquariums began as a class project on new business development. Now that the visionaries behind the idea have graduated, they want to explore their business idea and see if the concept could...
-
3. Modify the program of Example 05 so that, it takes and prints values using the following two functions respectively. void get (double *&a, int& n); void print (double *a, int n); 4. Following is a...
-
A company will be financing its operations with and a capital budget is P40,000,000 and a debt-to-equity ratio of 1. The interest rate on company's debt is 10%. The expected return on equity by the...
-
Fill in the blank to correctly complete each sentence. Any point that lies on the x-axis has y-coordinate equal to ____________.
-
Graph one period of each function. y = 4 cos x
-
Use Figs. 45 and 46 to answer the following questions. a. Would the bonding MO in HF place greater electron density near the H or the F atom? Why? b. Would the bonding MO have greater fluorine 2p...
-
The diatomic molecule OH exists in the gas phase. OH plays an important part in combustion reactions and is a reactive oxidizing agent in polluted air. The bond length and bond energy have been...
-
What is delocalized bonding, and what does it explain? Explain the delocalized bonding system in C6H6 (benzene) and SO2.
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


