The units of parts per million (ppm) and parts per billion (ppb) are commonly used by environmental
Question:
The units of parts per million (ppm) and parts per billion (ppb) are commonly used by environmental chemists. In general, 1 ppm means 1 part of solute for every 106 parts of solution. (Both solute and solution are measured using the same units.) Mathematically, by mass: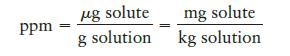
In the case of very dilute aqueous solutions, a concentration of 1.0 ppm is equal to 1.0 mg of solute per 1.0 mL of solution, which equals 1.0 g of solution. Parts per billion is defined in a similar fashion. Calculate the molarity of each of the following aqueous solutions.
a. 5.0 ppb Hg in H2O
b. 1.0 ppb CHCl3 in H2O
c. 10.0 ppm As in H2O
d. 0.10 ppm DDT (C14H9Cl5) in H2O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





