Three mechanisms for the reaction NO 2 (g) + CO(g) CO 2 (g) + NO(g) have
Question:
Three mechanisms for the reaction NO2(g) + CO(g) → CO2(g) + NO(g) have been proposed: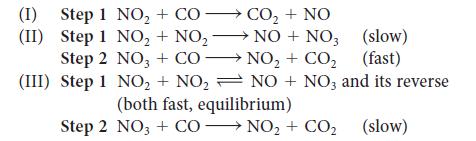
Which mechanism agrees with the following rate law: Rate = kr[NO2]2? Explain your reasoning.
Transcribed Image Text:
Step 1 NO₂ + Step 1 Step 2 (III) Step 1 (1) (II) Step 2 COCO₂ + NO NO + NO3 NO₂ CO₂ NO+ NO3 and its reverse NO₂ + NO₂ NO3 + CO- NO₂ + NO₂ (both fast, equilibrium) (slow) (fast) NO3 + CONO₂+ CO₂ NO₂ + CO₂ (slow)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
If mechanism I were correct the rate law would be rate k ...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In addition to vision system using visual light, scientist has invented new systems with capabilities beyond human visions. Discuss five latest technologies that able to map the unseen world and...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The reaction 2NO(g) + O2(g) 2NO2(g) exhibits the rate law Rate = k[NO]2[O2] Which of the following mechanisms is consistent with this rate law? a. NO + O2 NO2 + O Slow O + NO NO2 Fast b. NO + O2 ...
-
What is the maximum height above ground that a projectile of mass 0.790 kg, launched from ground level, can achieve if you are able to give it an initial speed of 80.3 m/s?
-
Explain how it might be possible to build an OS that uses full segmentation, but has no file system.
-
Passengers in an auto traveling at 16.0 m/s toward the east hear a siren frequency of 950 Hz from an emergency vehicle approaching them from behind at a speed (relative to the air and ground) of 40.0...
-
PR 5-1 Should the consolidated financial statements include the subsidiarys retained earnings at the acquisition date?
-
Pineapple Motor Company manufactures two types of specialty electric motors, a commercial motor and a residential motor, through two production departments, Assembly and Testing. Presently, the...
-
Kim invested her savings in a GIC that pays 4% interest. If the rate of inflation is 2% and her marginal tax rate is 22% what is her real after-tax rate of return?
-
Kenmare Architects Ltd. (KAL) was incorporated and commenced operations on January 1, 2014. Sheila Kenmare, the company's only employee, consults with various clients and uses expensive equipment to...
-
Dinitrogen pentoxide, N 2 O 5 , decomposes by a first-order reaction. What is the initial rate of decomposition of N 2 O 5 when 3.45 g of N 2 O 5 is confined in a container of volume 0.750 L and...
-
For the first-order reaction A 3 B + C, when [A] 0 = 0.015 mol L 1 , the concentration of B increases to 0.018 mol L 1 in 3.0 min. (a) What is the rate constant for the reaction expressed as the...
-
Blackburn Appliance Center accumulates the following cost and net realizable value data at December 31. Compute the lower of cost or net realizable value valuation for the companys total inventory....
-
A parent acquires all of the stock of a subsidiary for $40 million in cash. The subsidiarys books report the following account balances at the date of acquisition (in trial balance format)....
-
1. Given: The sign for the Inn of the Prancing Pony in Bree-yes, it comes in pints-is fixed on the end of a beam of length 5L. If the sigh deflects too much then Gandalf will hit his head when he...
-
Q21) Add positive and negative charges as shown in the diagram below. Use the arrows of the simulation to guide you in drawing continuous electric field lines around and in between the three charges....
-
When 10.1 g CaO is dropped into a styrofoam coffee cup containing 157 g H2O at 18.0C, the temperature rises to 35.8C. Calculate the enthalpy change of the following reaction in kJ/mol CaO. Assume...
-
4-12. Sometimes heterogeneous chemical reactions take place at the walls of tubes in which reactive mixtures are flowing. If species A is being consumed at a tube wall because of a chemical reaction,...
-
A plant physiologist conducted an experiment to determine whether mechanical stress can retard the growth of soybean plants. Young plants were randomly allocated to two groups of 13 plants each....
-
Independent random samples of sizes n1 = 30 and n2 = 50 are taken from two normal populations having the means 1 = 78 and 2 = 75 and the variances 21 = 150 and 22 = 200. Use the results of Exercise...
-
Identify which arrow-pushing pattern is utilized in the following step:
-
For each of the following multistep reactions, read the curved arrows and identify the sequence of arrowpushing patterns: a. b. c. d. e. :OH 0=s=0. :H 0=s=0: o=s=0:
-
The following two reactions will be explored in different chapters. Yet, they are very similar. Identify and compare the sequences of arrow-pushing patterns for the two reactions. Reaction 1 Reaction...
-
A contractor constructed a house for resale, which was sold immediately. For tax purposes, this is an example of A) capital income. B) business income. C) other income. D) property income.
-
You invest $100 in a risky asset with an expected rate of return of 0.12 and a standard deviation of 0.15 and a T-bill with a rate of return of 0.05. What percentages of your money must be invested...
-
Nanometrics, Inc., has a beta of 3.43. If the market return is expected to be 13.50 percent and the risk-free rate is 7.00 percent, what is Nanometrics required return? (Round your answer to 2...

Study smarter with the SolutionInn App


