Use data from Tables 6C.1 and 6C.2 to place the following acids in order of increasing strength:
Question:
Use data from Tables 6C.1 and 6C.2 to place the following acids in order of increasing strength: HNO2, HClO2, +NH3OH, (CH3)2NH2+.
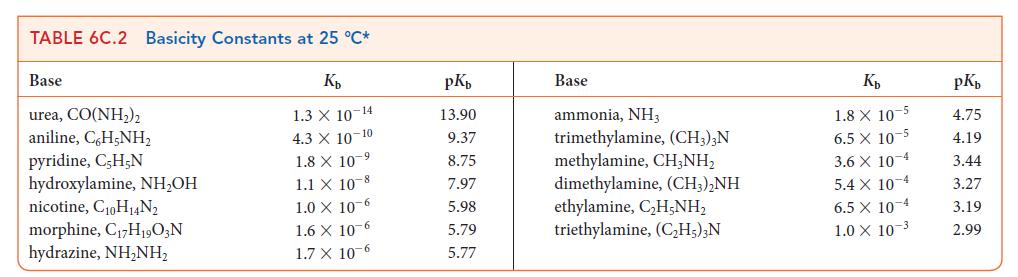
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* K₂ 3.0 X 10-1 2.0 X 10-1 1.7 X 10-1 1.5 X 1.0 X 10-2 Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H,SO3H iodic acid, HIO3 sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H3PO4 chloroacetic acid, CH₂ClCOOH lactic acid, CH₂CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF 10 2 7.6 X 10-3 1.4 x 10-3 8.4 X 10 4 4.3 X 10-4 3.5 x 10-4 pKa 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H₂COOH acetic acid, CH,COOH carbonic acid, H₂CO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3¹ hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO K₂ 1.8 X 10 4 6.5 x 10-5 1.8 X 10-5 4.3 X 107 3.0 X 108 2.0 × 10-⁹ 7.2 X 10-10 4.9 X 10-10 1.3 X 10-10 2.3 X 10-11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
CH32NH 1400 3...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use data from Tables 6C.1 and 6C.2 to place the following acids in order of increasing strength: HCOOH, (CH 3 ) 3 NH + , N 2 H 5 + , HF. TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0 X 10-...
-
a. Place the following acids in order of strength, starting with the strongest acid first. CH 3 CH 2 COOH CH 3 CCl 2 COOH CH 3 CHClCOOH b. Explain why ethanoic acid is a stronger acid than ethanol....
-
Use Paulings rules to place the following acids in order of increasing acid strength: HNO 2 , H 2 SO 4 , HBrO 3 , and HClO 4 in a nonlevelling solvent.
-
Metro Credit Union in Charlottetown, Prince Edward Island, loaned $90,000 to David Mann on a six-month, 8% note. Record the following for Metro Credit Union: a. Lending the money on March 6. b....
-
Effect of management evaluation criteria on EOQ model. Computers 4 U is an online company that sells computers to individual consumers. The annual demand for one model that will be shipped from the...
-
Suppose we put A = 0 in Eq. 38-17 and relabeled B as 0. (a) What would the resulting wave function then describe? (b) How, if at all, would Figure be altered?
-
Clearly explain the correspondence between an original scatter plot of the data and a plot of the residuals versus fitted values.
-
Edwards Construction currently has debt outstanding with a market value of $70,000 and a cost of 8%. The company has EBIT of $5600 that is expected to continue in perpetuity. Assume there are no...
-
what is the best discription of of a retail insurance certificacertification
-
A restaurant serves 30 guests in four hours and 3 servers (labor), the labor rate is $20/hour/server, and consumes $500 worth of food and other raw materials, and $50 of energy. Calculate...
-
A combustion analysis of 1.200 g of an anhydrous sodium salt gave 0.942 g of CO 2 , 0.0964 g of H 2 O, and 0.246 g of Na. The molar mass of the salt is 112.02 g mol 1 . (a) What is the chemical...
-
Calculate the molar solubility of each substance in its respective solution: (a) Silver chloride in 0.20 m NaCl(aq); (b) Mercury(I) chloride in 0.150 m NaCl(aq); (c) Lead(II) chloride in 0.025 m CaCl...
-
Answer Problems 1825 using the following probability tree: Start .4 .6 A A'- .2 .8 .3 7 - B B' B B'
-
Solve (c) 8 WI n=1 5 cos n N5
-
- Pierce Company reported net income of $160,000 with income tax expense of $19,000 for 2020. Depreciation recorded on buildings and equipment amounted to $80,000 for the year. Balances of the...
-
ABC Company had the following results as of 12/31/2020: ABC's hurdle rate is 10% CONTROLLABLE REVENUE CONTROLLABLE COST CONTROLLABLE ASSETS CONTROLLABLE INCOME 21. What is the division's margin? A....
-
A gray kangaroo can bound across a flat stretch of ground with each jump carrying it 10 m from the takeoff point. If the kangaroo leaves the ground at a 20 angle, what are its (a) takeoff speed and...
-
Since 1900, many new theories in physics have changed the way that physicists view the world. Create a presentation that will explain to middle school students why Quantum Mechanics is important, how...
-
Suppose that a medical test has a 92% chance of detecting a disease if the person has it (i.e., 92% sensitivity) and a 94% chance of correctly indicating that the disease is absent if the person...
-
A manufacturer can sell product 1 at a profit of $20 per unit and product 2 at a profit of $40 per unit. Three units of raw material are needed to manufacture one unit of product 1, and six units of...
-
Identify the reactants you would use to prepare each of the following azo dyes via an azo coupling reaction: (a) (b) (c) N- !i -NH2 S SO,H -NO2
-
Starting with benzene and isopropyl chloride, show how you would prepare the following compound: N- -NH2 O,N-
-
Draw the product obtained when the diazonium salt formed from aniline is treated with each of the following compounds: (a) Aniline (b) Phenol (c) Anisole (methoxybenzene)
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


