Use data from Tables 6C.1 and 6C.2 to place the following acids in order of increasing strength:
Question:
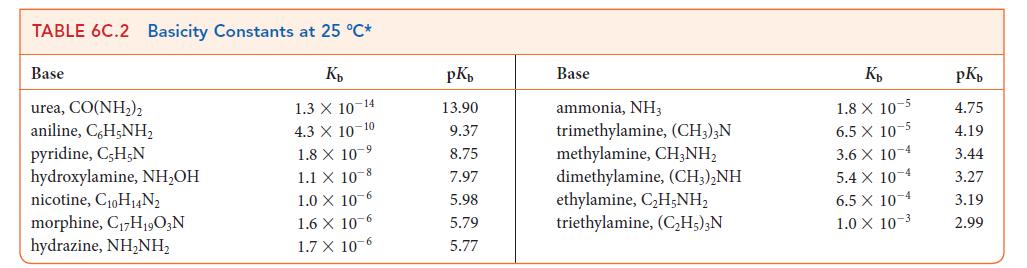
Use data from Tables 6C.1 and 6C.2 to place the following acids in order of increasing strength: HCOOH, (CH3)3NH+, N2H5+, HF.
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0 X 10- 1.7 X 10-1 Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C6H,SO3H iodic acid, HIO3 sulfurous acid, HSO3 chlorous acid, HClO phosphoric acid, H3PO4 chloroacetic acid, CHCICOOH lactic acid, CHCH(OH)COOH nitrous acid, HNO hydrofluoric acid, HF 1.5 X 10 2 1.0 X 10-2 7.6 X 10-3 1.4 X 10-3 8.4 X 10 4 4.3 X 10-4 3.5 X 10-4 pKa 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,HCOOH acetic acid, CH,COOH carbonic acid, HCO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3 hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO K 1.8 X 10-4 6.5 X 10-5 1.8 X 10-5 4.3 X 10-7 3.0 X 10 8 2.0 10-9 7.2 X 10-10 4.9 X 10-10 1.3 X 10-10 2.3 X 10-11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
Decreasing pKa will correspond t...View the full answer

Answered By

Divya Munir
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use data from Tables 6C.1 and 6C.2 to place the following acids in order of increasing strength: HNO 2 , HClO 2 , +NH 3 OH, (CH 3 ) 2 NH 2 + . TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0 X...
-
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: C 10 H 14 N 2 (nicotine), ClO 2 , (CH 3 ) 3 N, HSO 3 . TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X...
-
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: F , NH 3 , CH 3 CO 2 , C 5 H 5 N (pyridine). TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0...
-
Is land allowed to be depreciated? Why or why not?
-
New equipment purchase, income taxes Annas Bakery plans to purchase a new oven for its store. The oven has an estimated useful life of 4 years. The estimated pretax cash flows for the oven are as...
-
The breaking strength X of a certain rivet used in a machine engine has a mean 5000 psi and standard deviation 400 psi. A random sample of 36 rivets is taken. Consider the distribution of X, the...
-
In Chapter 7, we will learn to split the data set into a training data set and a test data set. To test whether there exist unwanted differences between the training and test set, which hypothesis...
-
Chilczuk, S.A., of Gdansk, Poland, is a major producer of classic Polish sausage. The company uses a standard cost system to help control costs. Manufacturing overhead is applied to production on the...
-
9 ) According to the FASB, how should unrealized gains on the investment portfolio of a not - for - profit organization be recognized? A ) Not recognized. B ) Reported in the net asset section of the...
-
Government actions such as price floors and price ceilings can actually increase unemployment and reduce market efficiency. True or False
-
The pH of several solutions was measured in a hospital laboratory; convert each of the following pH values into the molar concentration of H 3 O + ions: (a) 4.8 (the pH of a urine sample); (b) 0.7...
-
Calculate the molar solubility of each substance in its respective solution: (a) Silver iodide in 0.020 m NaI(aq); (b) Calcium carbonate in 2.3 * 10 4 m Na 2 CO 3 (aq); (c) Lead(II) fluoride in 0.21...
-
Find the limit or show that it does not exist. lim tan-(In x)
-
Mail - Jame Mail - Jame x a Amazon.co x a Amazon.co. X https://gpt x _ Calendar - - C * gptc.blackboard.com/webapps/blackboard/content/contentWrapper.jsp?content_id=_1846554 GEORGIA PIEDMONT...
-
Prepare a partnership return and the appropriate K-1s for W & M Partnership. William Winston (SSN: 226-00-4265) lives at 53 Mantis Road, Your City, Your State - Your Zip. He operates Lovely Lady...
-
A new store is opening in Rock Spring, with 175,000 available square feet. Each department must have at least 17,000 square feet and no department can have more than 24% of the total retail floor...
-
Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together with comparative data for the year ended December 31, 2021. From the statement of cash flows...
-
Stockholders of Sarasota Company, Riverbed Company, and Pronghorn Company are considering alternative arrangements for a business combination. Balance sheets and the fair values of each company's...
-
Calculate the mean, µY, of the random variable Y from Exercise 3.5.7. Exercise 3.5.7 The prevalence of mild myopia (nearsightedness) in adults over age 40 is 25% in the U.S. Suppose four adults...
-
The Cholesterol Level data sets give cholesterol levels of heart attack patients. Cholesterol measures are taken 2, 4, and 14 days aft er a patient has suffered a heart attack. Is there a significant...
-
Propose an efficient synthesis for each of the following transformations. (a) (b) (c) (d) OEt EtO
-
The product of an aldol condensation is an α,β unsaturated ketone which is capable of undergoing hydrogenation to yield a saturated ketone. Using this technique, identify...
-
Identify the reagents you would use to convert cyclohexanone into each of the following compounds. (a) (b) (c) (d) (e) (f) (g)
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...
-
A section 83(b) election creates ordinary income at the time of the grant. Ture or False

Study smarter with the SolutionInn App


