Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength:
Question:
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: C10H14N2 (nicotine), ClO2, (CH3)3N, HSO3–.
Transcribed Image Text:
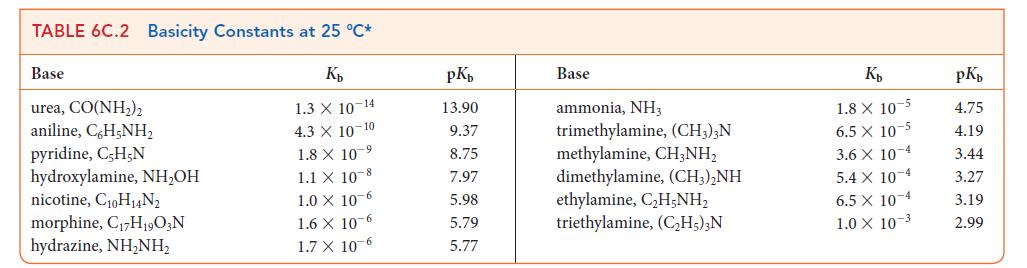
TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0 X 10-1 1.7 X 10-1 1.5 X 1.0 X 10-2 Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C6H5SO3H iodic acid, HIO3 sulfurous acid, HSO3 chlorous acid, HClO phosphoric acid, H3PO4 chloroacetic acid, CHCICOOH lactic acid, CHCH(OH)COOH nitrous acid, HNO hydrofluoric acid, HF 10 2 7.6 x 10-3 1.4 X 10-3 8.4 X 10 4 4.3 X 10-4 3.5 x 10-4 pKa 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,HCOOH acetic acid, CH,COOH carbonic acid, HCO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3 hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO K 1.8 X 10 4 6.5 X 10-5 1.8 X 10-5 4.3 X 107 3.0 X 108 2.0 10- 7.2 X 10-10 4.9 X 10-10 1.3 X 10-10 2.3 X 10-11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Decreasing pK will correspond to in...View the full answer

Answered By

Daniel Kimutai
I am a competent academic expert who delivers excellent writing content from various subjects that pertain to academics. It includes Electronics engineering, History, Economics, Government, Management, IT, Religion, English, Psychology, Sociology, among others. By using Grammarly and Turnitin tools, I make sure that the writing content is original and delivered in time. For seven years, I have worked as a freelance writer, and many scholars have achieved their career dreams through my assistance.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: F , NH 3 , CH 3 CO 2 , C 5 H 5 N (pyridine). TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0...
-
Arrange the following bases in order of increasing strength on the basis of the pK a values of their conjugate acids, which are given in parentheses: (a) Aniline (4.63; see Exercise 6C.12); (b)...
-
Arrange the following bases in order of increasing strength on the basis of the pK a values of their conjugate acids, which are given in parentheses: (a) Ammonia (9.26); (b) Methylamine (10.56); (c)...
-
Allocation of Common Costs. The cities of Albany, Troy and Schenectady are considering the implementation of a new program to handle disposal of hazardous waste to comply with a new, more stringent...
-
Draw the structures for these compounds: (a) (Z)-Oct-3-en-2-one (b) 3-Ethylheptanal (c) 2, 4-Pentadienal (d) 3, 4-Dimethylbenzaldehyde (e) 1-Phenyl-1-propanone (f) 2, 2, 6, 6-Ttramythleyelohexanone
-
Understand the importance of good communications on projects and the need to develop soft skills, especially for IT project managers and their teams? LO.1
-
From a recent production run of compact fluorescent (CF) bulbs, you select a random sample of 10 bulbs. Using an accelerated bulb life simulation, you find that the average life for the bulbs in the...
-
Ford wants to raise debt by issuing bonds. They were informed by their investment banking consultant that they would have to pay a commission of 1.0% of the selling price on new issues. The company...
-
Identifying financing, investing, and operating transactions Required For a company like Canadian Tire Corporation, provide two examples of transactions that you would classify as financing,...
-
Calculate the molar concentrations of H 2 SO 3 , HSO 3 , SO 3 2 , H 3 O + , and OH present in 0.125 m H 2 SO 3 (aq).
-
Calculate the molar solubility of each of the following sparingly soluble compounds in its respective solution: iron(III) hydroxide at (a) pH = 11.0; (b) pH = 3.0; iron(II) hydroxide at (c) pH = 8.0;...
-
Why do 8-ounce boxing gloves hit harder than 16-ounce gloves?
-
Rosita Flores owns Rosita's Mexican Restaurant in Tempe, Arizona. Rosita's is an affordable restaurant near campus and several hotels. Rosita accepts cash and checks. Checks are deposited...
-
Your second task will require you to recover a payload from the conversation. Just need 2.3. Need you to explain step by step, and concept by concept if possible. Use wireshark. Tell me your answer...
-
2. Supply for art sketchbooks at a price of $p per book can be modelled by P <10 S(p) = = textbooks. p3+p+3 p 10 (a) What is the producer revenue at the shutdown point? (b) What is the producer...
-
Patterson Company produces wafers for integrated circuits. Data for the most recent year are provided: Expected Consumption Ratios Activity Driver Wafer A Wafer B Inserting and sorting process...
-
The elementary gas-phase reaction 2A + B C+D is carried out isothermally at 450 K in a PBR with no pressure drop. The specific reaction rate was measured to be 2x10-3 L/(mol-min-kgcat) at 50C and the...
-
Consider taking a random sample of size 25 from a population in which 42% of the people have type A blood. What is the probability that the sample proportion with type A blood will be greater than...
-
Suppose Green Network Energy needs to raise money to finance its new manufacturing facility, but their CFO does not think the company is financially capable of making the periodic interest payments...
-
At 39.9C, a solution of ethanol (x 1 = 0.9006, P * 1 = 130.4 Torr) and isooctane (P * 2 = 43.9 Torr) forms a vapor phase with y 1 = 0.6667 at a total pressure of 185.9 Torr. a. Calculate the activity...
-
Calculate the solubility of H 2 S in 1 L of water if its pressure above the solution is 2.75 Pa. The density of water at this temperature is 997kg m 3 .
-
The binding of NADH to human liver mitochondrial isozyme was studied [Biochemistry 28 (1989): 5367] and it was determined that only a single binding site is present with K = 2.0 10 7 M 1 . What...
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


