Arrange the following bases in order of increasing strength on the basis of the pK a values
Question:
Arrange the following bases in order of increasing strength on the basis of the pKa values of their conjugate acids, which are given in parentheses:
(a) Aniline (4.63; see Exercise 6C.12);
(b) 2-hydroxyaniline (4.72);
(c) 3-hydroxyaniline (4.17);
(d) 4-hydroxyaniline (5.47). Is there a simple pattern of strengths?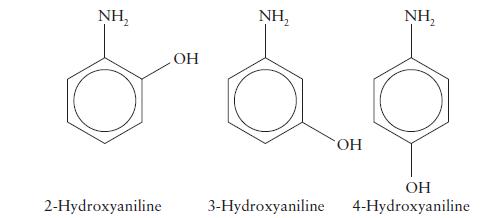
Exercise 6C.12
The value of pKb for aniline is 9.37 and that for 4-chloroaniline is 9.85. Which is the stronger base? Account for the difference in strength.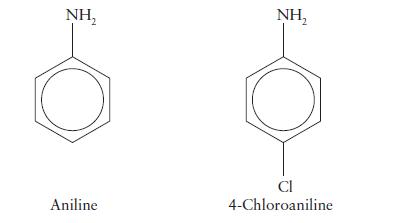
Transcribed Image Text:
NH OH NH 2-Hydroxyaniline 3-Hydroxyaniline OH NH OH 4-Hydroxyaniline
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The higher the pKa of ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Arrange the following bases in order of increasing strength on the basis of the pK a values of their conjugate acids, which are given in parentheses: (a) Ammonia (9.26); (b) Methylamine (10.56); (c)...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: C 10 H 14 N 2 (nicotine), ClO 2 , (CH 3 ) 3 N, HSO 3 . TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X...
-
Hrishi is a senior executive for a large manufacturing company in Mississauga Ontario where he has been employed for the past 10 years and his annual salary is $250,000 including bonus. He is 47...
-
Analysis of growth, price-recovery, and productivity components (continuation of 13-31) Suppose that during 2009, the market for ZP98 grew 8%. All increases in market share (that is, sales increases...
-
A radar transmitter Z is fixed to a reference frame S' that is moving to the right with speed v relative to reference frame S (Figure). A mechanical timer (essentially a clock) in frame S', having a...
-
What signals does the department send by making project manager the highest position in the department? The IT Department at Hamelin Hospital Hamelin Hospital is a large (700-bed) regional hospital...
-
One of the products of the G.A. Tanner Company is a special kind of toy that provides an estimated unit profit of $3. Because of a large demand for this toy, management would like to increase its...
-
The higher the market interest rate compared to the stated rate, the lower the bond issue price will be. True or false
-
A triage system has been proposed for the ER described in Exercise 3.4. Under the proposed triage plan, entering patients will be registered as before. They will then be quickly examined by a nurse...
-
Draw the Lewis structure or symbol for each of the following species and identify each one as a Lewis acid or Lewis base: (a) SO; (b) I; (c) CH3S (the C atom is the central atom); (d) NH; (e) NO.
-
Topic 6C discusses the relationship between molecular structure and the strengths of acids. The same ideas can be applied to bases. (a) Explain the relative strengths of the Brnsted bases OH , NH 2 ...
-
Refer to Problem 10.13. a) If the allowance factor is 15%, what is the standard time for this operation? b) If the allowance factor is 18% and the performance rating is now 90%, what is the standard...
-
A production Edgeworth Box, with origins indicated for the inputs of capital, K , and labor, L , into production of goods X and Y .Eight isoquants are shown, reflecting standard...
-
For 2014, Nichols, Inc., had sales of 150,000 units and production of 200,000 units. Other information for the year included: Direct manufacturing labor 187,500 Variable manufacturing overhead...
-
reading the following statement and decide whether you agree or disagree with the statement: "The free market system is the best economic system since it is the most efficient and solves basic...
-
find the net presbf value of the project ? present value index? Net present value A project has estimated annual net cash flows of $11,250 for 10 years and is estimated to cost $42,500. Assume a...
-
Calculate the ICER for the new treatment, without adjusting for the health utility index. Assuming the $50K benchmark*, as a clinical decision maker or health policy advisor, would you recommend...
-
In an experiment to study the regulation of insulin secretion, blood samples were obtained from seven dogs before and after electrical stimulation of the vagus nerve. The following values show, for...
-
Give the structural formulas of the alkenes that, on ozonolysis, give: a. (CH3)2C=O and CH2=O b. Only (CH3CH2)2C=O c. CH3CH=O and CH3CH2CH=O d. O=CHCH2CH2CH2CH=O
-
DexonTM (below) is a polyester that is spun into fibers and used for surgical stitches that dissolve over time, eliminating the need for a follow-up procedure to remove the stitches. The ester...
-
Draw the structure of the polymer produced when the following two monomers are allowed to react with each other: CI CI
-
Identify what monomers you would use to produce the following polymer: Z Z Z Z
-
4) Read the following case carefully and answer the given questions. You have been the finance director of a clothing retailer for ten years. The companys year end is 31st December 2019, and you are...
-
all of the other problems here on chegg don't describe right on how they god the answer. can you make it step by step math to show how you got what and from where and each number to get the answer...
-
D Required information The following Information applies to the questions displayed below.) Diego Company manufactures one product that is sold for $76 per unit in two geographic regions-the East and...

Study smarter with the SolutionInn App


