Topic 6C discusses the relationship between molecular structure and the strengths of acids. The same ideas can
Question:
Topic 6C discusses the relationship between molecular structure and the strengths of acids. The same ideas can be applied to bases.
(a) Explain the relative strengths of the Brønsted bases OH–, NH2 –, and CH3 – (see Table 6C.3).
(b) Explain why NH3 is a weak base in water, but PH3 forms essentially neutral solutions.
(c) If you were ranking the species in (a) or (b) as Lewis bases, would your rankings be the same or different? Explain your reasoning.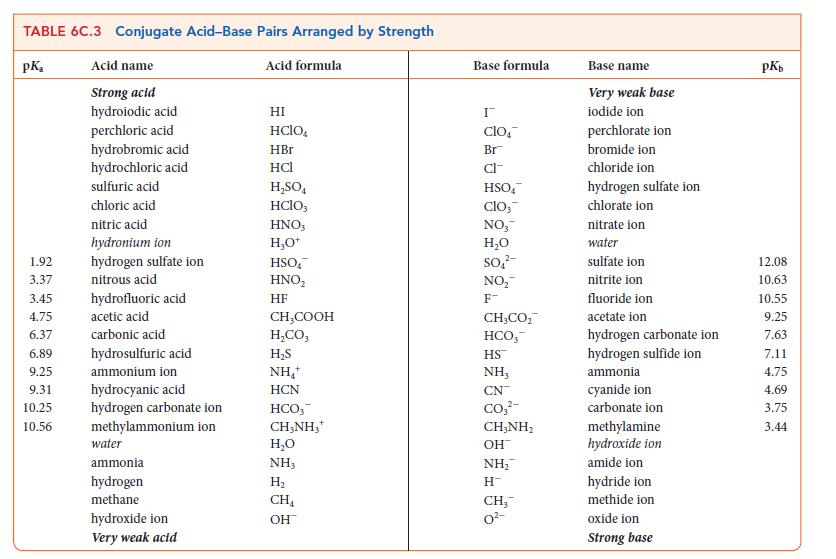
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





