Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength:
Question:
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: F–, NH3, CH3CO2–, C5H5N (pyridine).
Transcribed Image Text:
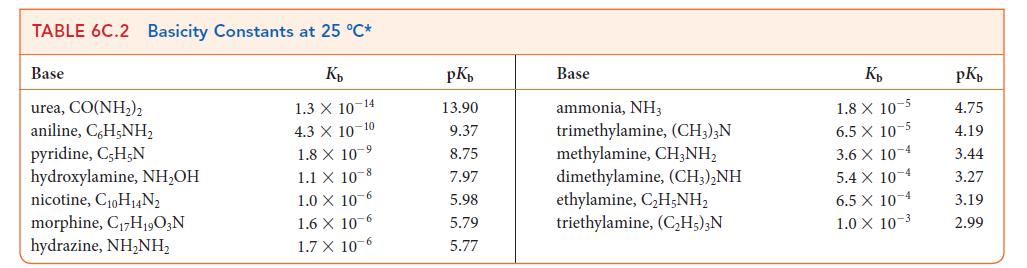
TABLE 6C.1 Acidity Constants at 25 °C* K₂ 3.0 X 10-1 2.0 X 10-1 1.7 X 10-1 1.5 X 1.0 X 10-2 Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C6H5SO3H iodic acid, HIO3 sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H3PO4 chloroacetic acid, CH₂ClCOOH lactic acid, CH₂CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF 10 2 7.6 X 10-3 1.4 X 10-3 8.4 X 10 4 4.3 X 10-4 3.5 x 10-4 pKa 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H₂COOH acetic acid, CH,COOH carbonic acid, H₂CO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3¹ hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO K₂ 1.8 X 10 4 6.5 X 10-5 1.8 X 10-5 4.3 X 107 3.0 X 108 2.0 × 10-⁹ 7.2 X 10-10 4.9 X 10-10 1.3 X 10-10 2.3 X 10-11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
F 1400 345 ...View the full answer

Answered By

Lamya S
Highly creative, resourceful and dedicated High School Teacher with a good fluency in English (IELTS- 7.5 band scorer) and an excellent record of successful classroom presentations.
I have more than 2 years experience in tutoring students especially by using my note making strategies.
Especially adept at teaching methods of business functions and management through a positive, and flexible teaching style with the willingness to work beyond the call of duty.
Committed to ongoing professional development and spreading the knowledge within myself to the blooming ones to make them fly with a colorful wing of future.
I do always believe that more than being a teacher who teaches students subjects,...i rather want to be a teacher who wants to teach students how to love learning..
Subjects i handle :
Business studies
Management studies
Operations Management
Organisational Behaviour
Change Management
Research Methodology
Strategy Management
Economics
Human Resource Management
Performance Management
Training
International Business
Business Ethics
Business Communication
Things you can expect from me :
- A clear cut answer
- A detailed conceptual way of explanation
- Simplified answer form of complex topics
- Diagrams and examples filled answers
4.90+
46+ Reviews
54+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: C 10 H 14 N 2 (nicotine), ClO 2 , (CH 3 ) 3 N, HSO 3 . TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X...
-
Arrange the following bases in order of increasing strength on the basis of the pK a values of their conjugate acids, which are given in parentheses: (a) Aniline (4.63; see Exercise 6C.12); (b)...
-
Arrange the following bases in order of increasing strength on the basis of the pK a values of their conjugate acids, which are given in parentheses: (a) Ammonia (9.26); (b) Methylamine (10.56); (c)...
-
Christina is trying to save money in her bank account. She decides that she can deposit $1000 each month into the account. The account earns 2.3% interest each month. How much money will be in the...
-
Variance analysis, multiple products. Debbies Delight, Inc., operates a chain of cookie stores. Budgeted and actual operating data of its three Chicago stores for August 2009 are as follows: Debbies...
-
An investment firm offers its customers municipal bonds that mature after varying numbers of years. Given that the cumulative distribution function of T, the number of years to maturity for a...
-
A ________________ report is a reflective statement that documents important information learned from working on the project. a. final project b. lessons-learned c. project archive d. progress LO.1
-
An advertising executive wants to estimate the mean amount of time that consumers spend with digital media daily. From past studies, the standard deviation is estimated as 45 minutes. a. What sample...
-
Using the following information, compute cash ow from operating activities. Cash Inow (Outow) Cash received from sale of a building $5,600 Cash paid for interest (450) Cash paid to repurchase shares...
-
Aritzia Inc.s financial statements have been reproduced in Appendix A at the back of the textbook. Instructions a. Many companies use a calendar year for their financial statements. What does Aritzia...
-
Use the data in Appendix 2B to calculate E(U 4+ /U). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 HO F+2 e...
-
Calculate the pH and pOH of each of the following aqueous solutions of a strong acid or base: (a) 0.0146 m HNO 3 (aq); (b) 0.11 m HCl(aq); (c) 0.0092 m Ba(OH) 2 (aq); (d) 2.00 mL of 0.175 m KOH(aq)...
-
Consider the list of current and classic issues in accounting presented below (or others specified by your instructor). With your instructors help and guidance, form a team of students to investigate...
-
Admin Support Cereal Bars Square Foot 1,250 1,500 7,500 7,000 # of employees 14 11 42 59 # of machine batches 0 0 14 27 # of computers 17 21 35 30 Costs 32,000.32 21,740.21 The Support department...
-
Compare and contrast the differences between innovation and creativity. Does one lead to the other? If so, please explain. Why is innovation important? Who within the organization is responsible for...
-
Using the tables from Check your Consumer Surplus and Producer Surplus activities, find the equilibrium price and quantity in the market for cheese-stuffed jalapeno peppers. What is the total surplus...
-
We decided to use Gehan's two-stage design for this purpose. In the first stage, we will discard the new treatment if no patient out of n0 patients. Suppose the probability we can tolerate to discard...
-
Claude Haridge was involved in a demonstration. He threw a paint balloon at a bus and some of the paint flecks hits a nearby officer, so Haridge was transported to police cells. At the cells Special...
-
Refer to Exercise 5.4.12. Suppose that the biologist takes a random sample of size 50. Find the probability that fewer than 35 of the sampled mussels will be infected, using the normal approximation...
-
Rosalie owns 50% of the outstanding stock of Salmon Corporation. In a qualifying stock redemption, Salmon distributes $80,000 to Rosalie in exchange for one-half of her shares, which have a basis of...
-
Why is it not appropriate to use ionic radii from crystal structures to calculate G solvation of ions using the Born model?
-
Why do deviations from ideal behavior occur at lower concentrations for electrolyte solutions than for solutions in which the solute species are uncharged?
-
Why is the value for the dielectric constant for water in the solvation shell around ions less than that for bulk water?
-
Long-term liabilities are shown in two places in the business firm's balance sheet depending upon when the long-term liabilities are scheduled for payment. True False
-
Julio is single with 1 withholding allowance. He earned $1,025.00 during the most recent semimonthly pay period. He needs to decide between contributing 3% and $30 to his 401(k) plan. If he chooses...
-
Acquirer firm plans to launch a takeover of Target firm. The manager of Acquirer indicates that the deal will increase the free cash flow of the combined business by $13.6m per year forever. The beta...

Study smarter with the SolutionInn App


