Use the data in Appendix 2B to calculate E(U 4+ /U). 2B STANDARD POTENTIALS AT 25 C
Question:
Use the data in Appendix 2B to calculate E°(U4+/U).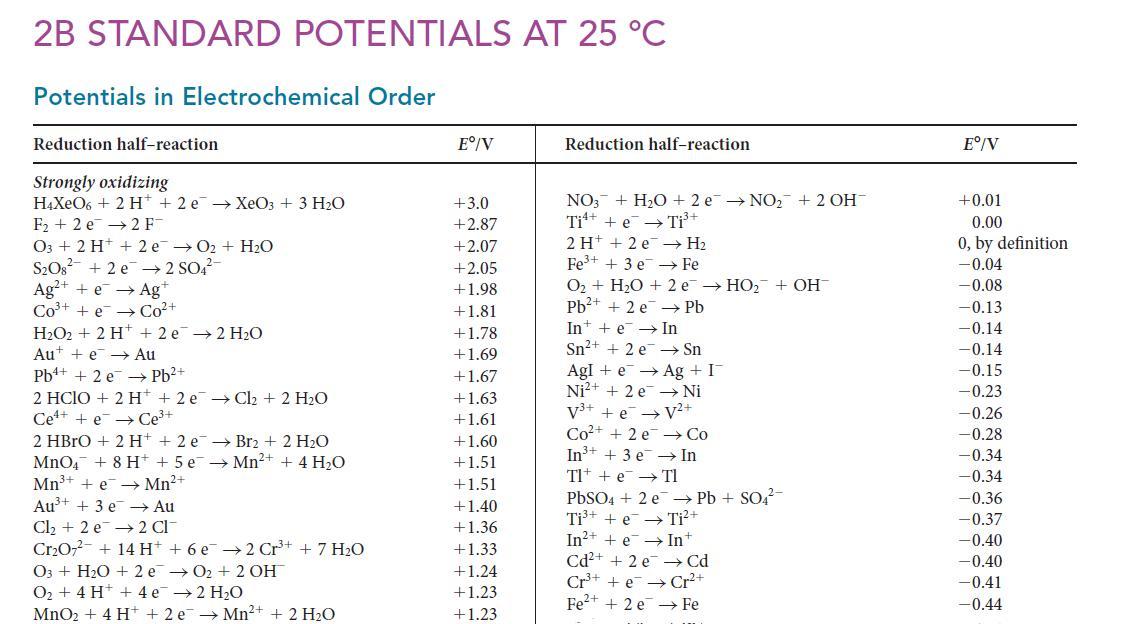
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e → XeO3 + 3 H₂O F₂+2 e 2 FT 03 + 2 H+ + 2 e → O₂ + H₂O S₂O8² +2e →2 SO4² Ag+ + e → Ag+ CO³+ +e→Co²+ H₂O2 + 2 H+2 e 2 H₂O Aut + e→→ Au Pb+ + 2e →→ Pb²+ 2 HCIO + 2 H+2 e → Cl₂ + 2 H₂O Cete Ce³+ 2 HBrO + 2 H+ + 2 e MnO4 + 8 H+ + 5e Mn³+ + e → Mn²+ Au³+ + 3 e → Au Cl₂ + 2 e 2 Cl Cr₂O7²- + 14 H+ + 6 e 2 Cr³+ + 7 H₂O → Br₂ + 2 H₂O →Mn²+ + 4 H₂O O3 + H₂O + 2e →O₂+ 2 OH O₂ + 4H+ + 4e¯¯ → 2 H₂O MnO₂ + 4H+ + 2e →Mn²+ + 2 H₂O Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 +1.23 Reduction half-reaction NO3 + H₂O +2 e NO₂ + 2 OH Ti + e Ti³+ 2 H+2e → H₂ Fe³+ + 3 e Fe O₂ + H₂O + 2 e → HO₂ + OH Pb²+ + 2 e Pb In + e → In Sn²+ + 2 e Sn Agle → Ag + I Ni²+ + 2 e Ni V³+ + eV²+ Co²+ + 2e → Co In³+ + 3 e → In Tl TIe PbSO4 + 2 e Pb + SO4²- Ti³++eTi²+ In²+ + e → Int Cd²+ + 2 e Cr³+ + Cr²+ Fe²+ + 2 e Fe Cd Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2B to calculate E(Ti 3+ /Ti). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 HO F +2e...
-
Use only the data in Appendix 2B to calculate the acidity constant of HClO in water. 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing...
-
K sp for Ni(OH) 2 is 6.5 * 10 18 . Use this value and data from Appendix 2B to calculate E for the half-reaction Ni(OH) 2 (s) + 2 e Ni(s) + 2 OH (aq). 2B STANDARD POTENTIALS AT 25 C Potentials in...
-
Duhail Complex averages about 15% of Bananas spoil before they can be sold. The manager purchases 200kg of bananas for 2.03QR per kilogram. What is the selling price if there is a 45% markup on the...
-
Market-share and market-size variances (continuation of 14-34). Debbies Delight attains a 10% market share based on total sales of the Chicago market. The total Chicago market is expected to be...
-
The proportion of people who respond to a certain mail-order solicitation is a continuous random variable X that has the density function(a) Show that P(0 (b) Find the probability that more than 1/4...
-
Which of the following is not a guideline to help improve time spent at meetings? a. Determine if a meeting can be avoided. b. Invite extra people who support your project to make the meeting run...
-
Enumclaw Brick, Inc., manufactures bricks using clay deposits on the companys property. Raw clays are blended and then extruded into molds to form unfired bricks. The unfired bricks are then stacked...
-
under common law which of the following is a basis for litigation against an auditor for failure to meet professional standards and responsibilities? Ordinary negligence Gross negligence Fraud All of...
-
Unexpected Indent /2 In Python, we increase the indentation level of our code to define a new block for statements like def. Indentation is expected to be consistent. The code below uses inconsistent...
-
Calculate the molar concentrations of H 2 CO 3 , HCO 3 , CO 3 2 , H 3 O + , and OH present in 0.0456 m H 2 CO 3 (aq).
-
Use data from Tables 6C.1 and 6C.2 to place the following bases in order of increasing strength: F , NH 3 , CH 3 CO 2 , C 5 H 5 N (pyridine). TABLE 6C.1 Acidity Constants at 25 C* K 3.0 X 10-1 2.0...
-
Add an additional student constructor to the university/student example to accept the university constructor argument by reference, rather than by pointer. For example, in addition to the constructor...
-
A carload of Hg-ore containing grains of cinnabar (86%Hg by mass; density = 8.19 g/cm3) and grains of basalt (containing no Hg; density=2.84 g/cm3) is to be sampled and analyzed for mercury. The...
-
CMS reviews acute IPPS and long-term care hospital (LTCH) records for payment purposes. Documentation and coding assignment must be accurate and specific. CMS contracts with Medicare Administrative...
-
Problem 2. x3+2x+1 f(x) = = 5-x 8H xx (4 points) Without graphing the function, find the limits lim f(x) and lim f(x) analyt- ically and show your work. Specify if the limits are - or +. (1 point)...
-
For change management, answer the following questions in detail, citing some industry examples: 1. What would you do if your manager requested you change your way of working on a project? 2. What do...
-
1.Sony has just released a new CD recording (okay, not new because we don't buy CDS) but anyway.Here is some cost and price information: CD Disc and Packaging (material and labor) $1.75/CD...
-
A fair coin is to be tossed 20 times. Find the probability that 10 of the tosses will fall heads and 10 will fall tails, (a) Using the binomial distribution formula. (b) Using the normal...
-
Velshi Printers has contracts to complete weekly supplements required by fortysix customers. For the year 2018, manufacturing overhead cost estimates total $600,000 for an annual production capacity...
-
Why does an increase in the ionic strength in the range where the DebyeHckel law is valid lead to an increase in the solubility of a weakly soluble salt?
-
What is the correct order of the following inert electrolytes in their ability to increase the degree of dissociation of acetic acid? a. 0.001m NaCl b. 0.001m KBr c. 0.10m CuCl 2
-
How does salting in affect solubility?
-
Transcribed image text
-
QUESTION 20 Assume a company reported the following results Sales Net operating income Average operating assets Margin Turnover Return on investment (ROI) 5300,000 2 $240.000 40% ? 2 What is the net...
-
2. Using the graph provided below, determine the fixed cost, the total variable cost, the variable cost per unit, and the TOTAL COST to produce 60 units. Fixed Cost ______________ Variable Cost...

Study smarter with the SolutionInn App


