Use the data in Appendix 2B to calculate E(Ti 3+ /Ti). 2B STANDARD POTENTIALS AT 25 C
Question:
Use the data in Appendix 2B to calculate E°(Ti3+/Ti).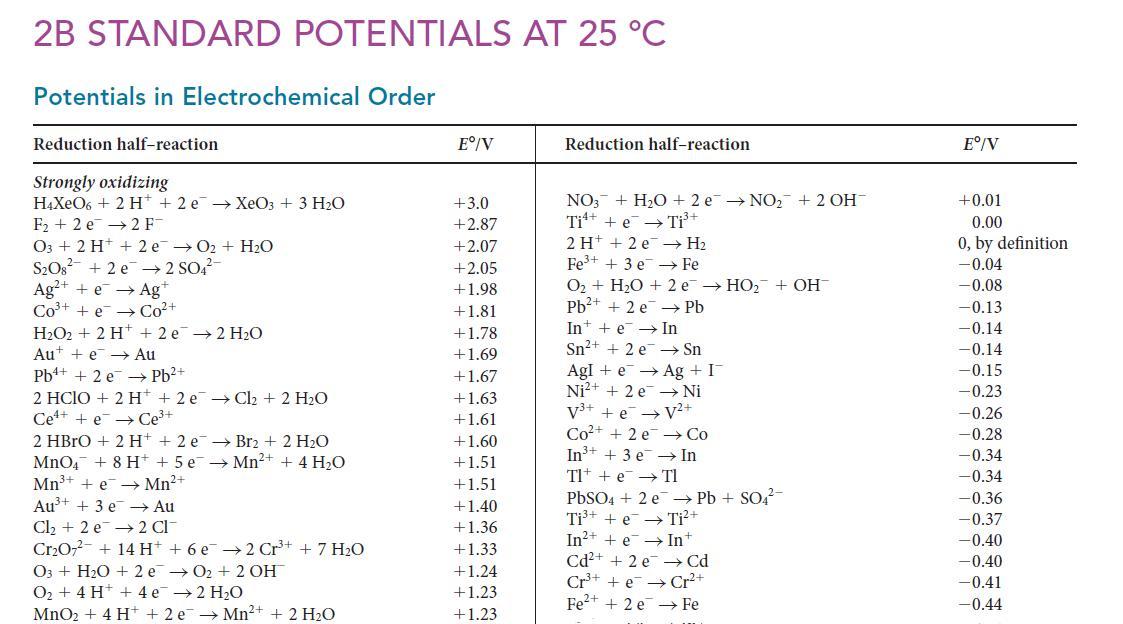
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 HO F +2e 2 FT 03 + 2 H+ + 2e O + HO SO8 +2e 2 SO4- Ag+ +eAg+ CO+ +eCo+ HO2 + 2 H+2e 2 HO Au + e Au Pb+ + 2e Pb+ 2 HCIO + 2 H + 2 e Cl + 2 HO Cee Ce+ 2 HBrO + 2 H+ + 2 e MnO4 + 8 H + 5 e Mn+ + e Mn+ Au+ + 3 e Au Cl + 2 e 2 Cl CrO7 + 14 H + 6 e2 Cr++ 7 HO Br + 2 HO Mn+ + 4 HO O3 + HO + 2eO+ 2 OH O + 4 H+ + 4e 2 HO MnO + 4 H+ + 2e Mn+ + 2 HO E/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +1.23 +1.23 Reduction half-reaction NO3 + HO + 2e NO + 2 OH Ti + e Ti+ 2 H+2e H Fe+ + 3 e Fe O + HO + 2 e HO + OH Pb+ +2 e Pb Int + e In Sn+ + 2 e Sn Agle Ag + I Ni+ + 2 e Ni V+ +ev+ Co+ +2e Co In+ + 3 e In Tl + e Tl PbSO4 + 2 e Pb + SO4- Ti+ + e Ti+ In+ + e Int Cd+ + 2 e Cd Cr+ + eCr+ Fe+ + 2 e Fe E/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41 -0.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The appropriate halfreactions are Ti e Ti E037 A Ti 2 e Ti E 163 B A and B a...View the full answer

Answered By

Evans Cherono
I am an Information Technology Graduate and willing to work on any computer science or IT work to ensure I do my best all the time.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use only the data in Appendix 2B to calculate the acidity constant of HClO in water. 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing...
-
Use the data in Appendix 2B to calculate E(U 4+ /U). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+ 2 e XeO3 + 3 HO F+2 e...
-
K sp for Ni(OH) 2 is 6.5 * 10 18 . Use this value and data from Appendix 2B to calculate E for the half-reaction Ni(OH) 2 (s) + 2 e Ni(s) + 2 OH (aq). 2B STANDARD POTENTIALS AT 25 C Potentials in...
-
Use the method of separation of variables to find the product solution of the PDES: (a) u + Uy=3u (b) xu = 2yuy
-
Allocation of common costs. Sunny Dunn, a self-employed consultant near Sacramento, received an invitation to visit a prospective client in Baltimore. A few days later, she received an invitation to...
-
Suggest a possible structure for a compound with the formula C9H10O that has the following IR spectrum and explain yourreasoning: 100 - 50 1706 em- 4000 3500 3000 2500 2000 1000 500 1500 Wavenamber...
-
Explain the elements of planning project communications and how to create a communications management plan? LO.1
-
The cost of Baxters inventory at the end of the year was $50,000. Due to obsolescence, the cost to replace the inventory was only $40,000. Net realizable valuewhat the inventory could be sold foris...
-
ProTech Company purchased a machine for $ 5 0 , 0 0 0 on January 1 , 2 0 1 8 . The company expects the service life of the machine to be 5 years. During that time, it is expected that the machine's...
-
Imagine we have created a Binary Search Tree by inserting the following sequence of integers into an empty Binary Search Tree (in this exact order): 10, 20 Provide a single positive integer that,...
-
Calculate the molar solubility of each of the following sparingly soluble compounds in its respective solution: iron(III) hydroxide at (a) pH = 11.0; (b) pH = 3.0; iron(II) hydroxide at (c) pH = 8.0;...
-
Which of the following reactions can be classified as reactions between Brnsted acids and bases? For those that can be so classified, identify the acid and the base. (a) KOH(aq) + CH3I(aq) CH3OH(aq)...
-
Bill Jackson worked during school and during the first 2 months of pis summer vacation. After factoring in his deductions and personal exemption as a single man, Bill found that he had a total...
-
Consider the following thermochemical equation: 2 Na 2 O 2 (s) + 2 H 2 O(l) 4 NaOH(s) + O 2 (g) H = -126 kJ Calculate the enthalpy change when 41.5 g of Na 2 O 2 with water?
-
Newton's Laws Introduction Problems
-
Mr. A and B agreed to start a business agreed to share profit and loss based the condition that will profit only when there is profit in excess of BD 10,000 this from of business is called as:...
-
L= {a'e"b"d' | i=1+m and l,m,n 20] a. Write at least 10 strings of the above language in increasing order of string length. b. Write Context Free Grammar (CFG) for the above language.
-
The Beta Co. shows the following results of operation on Dec. 31. Variable cost Fixed costs Direct materials P512,500 Direct labor 575,000 Manufacturing overhead 400,000 P212,500 For the year then...
-
The heights of men in a certain population follow a normal distribution with mean 69.7 inches and standard deviation 2.8 inches.15 (a) If a man is chosen at random from the population, find the...
-
A summary of changes in Pen Corporation's Investment in Sam account from January 1, 2011, to December 31, 2013, follows (in thousands): ADDITIONAL INFORMATION 1. Pen acquired its 80 percent interest...
-
The data from Problem P9.20 can be expressed in terms of the molality rather than the mole fraction of Br2 . Use the data from the following table and a graphical method to determine the Henrys law...
-
The densities of pure water and ethanol are 997 and 789 kg m 3 , respectively. For x ethanol = 0.35, the partial molar volumes of ethanol and water are 55.2 and 17.8 10 3 L mol 1 , respectively....
-
Two liquids, A and B, are immiscible for x A = x B = 0.5, for T < 75.0????C and completely miscible for T > 75.0????C. Sketch the phase diagram, showing as much information as you can from these...
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


