Which of the following reactions can be classified as reactions between Brnsted acids and bases? For those
Question:
Which of the following reactions can be classified as reactions between Brønsted acids and bases? For those that can be so classified, identify the acid and the base.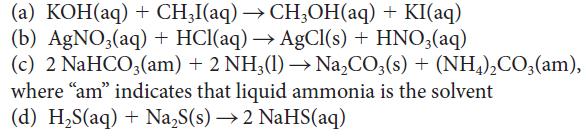
Transcribed Image Text:
(a) KOH(aq) + CH3I(aq) → CH3OH(aq) + KI(aq) (b) AgNO3(aq) + HCl(aq) → AgCl(s) + HNO3(aq) (c) 2 NaHCO3(am) + 2 NH3(1)→→ Na₂CO3(s) + (NH4)₂CO3(am), where "am" indicates that liquid ammonia is the solvent (d) H,S(aq) + Na,S(s) → 2 NaHS(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a KOHaq CH3Iaq CH3OHaq KIaq This reaction involves the transfer of a proton H from CH3I t...View the full answer

Answered By

Patrick Busaka
I am a result oriented and motivated person with passion for challenges because they provide me an opportunity to grow professionally.
5.00+
38+ Reviews
58+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
An investor wishes to analyse the effects of different compounding frequencies Suppose 1000 is invested for 1 year at an interest rate of 5 per annum compounded Assume there are 365 days in 1 year
-
Proteins are synthesized with a particular amino acid sequence through the translation of information encoded in messenger RNA by an RNAprotein complex called a ribosome. Amino acids are specified by...
-
1. Which one of the following is Not an assumption of the binomial distribution? The probability of the success, p, is constant. The probability of the success is equal to 0.5 in all trials. There...
-
Consider X[k] be the N-point DFT of an N-point sequence x[n]. (20 %) x [n] = {1, -2, 1, 3} x [n] = {0, 2, -1,0,0,4} (a) Determine the linear convolution x [n] * x [n] (5%) (b) Determine the...
-
Allocation of common costs. Mike and Ed are students at Berkeley College. They share an apartment that is owned by Ed. Ed is considering subscribing to an Internet provider that has the following...
-
Predict the important absorptions in the IR spectra of these compounds.
-
Describe how to manage communications, including communication technologies, media, and performance reporting? LO.1
-
On January 1, 2018, National Insulation Corporation (NIC) leased equipment from United Leasing under a finance lease. Lease payments are made annually. Title does not transfer to the lessee and there...
-
We want to acquire firm XYZ. Therefore, we need to estimate its value in order to negotiate the acquisition. XYZ's EBITDA is 5 million and it has no debt. XYZ's best comparable firms are ABC, DEF and...
-
Kerron owns a shop supplying fishing tackle. During March 2014 he returned goods to suppliers that had previously been purchased on credit. Prepare the following accounting records to record these...
-
Use the data in Appendix 2B to calculate E(Ti 3+ /Ti). 2B STANDARD POTENTIALS AT 25 C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 HO F +2e...
-
(a) Write balanced half-reactions for the redox reaction between sodium perchlorate and copper (I) nitrate in an acidic solution. (b) Write the balanced equation for the cell reaction and devise a...
-
Consider Problem 7.113. Estimate the heat transfer rate to the air, accounting for both the increase in the air temperature as it flows through the foam and the thermal resistance associated with...
-
Explain in details the reasons for your classifications. Classify the following processes as batch, continuous, or semibatch, and transient or steady- state. 1. A balloon is filled with air at a...
-
Question 5. A first responder drone of mass m slug is launched with a velocity vo ft/sec and constant engine force F from a level ground and moves vertically upward to discover a sense of life in a...
-
As part of the investigation of the collapse of the roof of a building. a testing laboratory is given all the available bolts that connected the steel structure at 3 different positions on the roof....
-
1) Baris Diary Co. has three product and divisions for production process of Milk, Yogurt and Cheese. Company's data show following resulst for 2014: Milk Yogurt Revenue 100.000TL 125.000TL Cheese...
-
Problem 11-4B (Algo) Prepare a statement of cash flows-indirect method (LO11-2, 11-3, 11-4, 11-5) The income statement, balance sheets, and additional information for Virtual Gaming Systems are...
-
Consider taking a random sample of size 14 from the population of students at a certain college and measuring the diastolic blood pressure each of the 14 students. In the context of this setting,...
-
The percentage of completion and completed contract methods are described in the FASB ASC. Search the codification to find the paragraphs covering these topics, cite them, and copy the results.
-
An ideal solution is formed by mixing liquids A and B at 298 K. The vapor pressure of pure A is 151 Torr and that of pure B is 84.3 Torr. If the mole fraction of A in the vapor is 0.610, what is the...
-
A solution is prepared by dissolving 45.2 g of a nonvolatile solute in 119 g of water. The vapor pressure above the solution is 22.51 Torr and the vapor pressure of pure water is 23.76 Torr at this...
-
A sample of glucose (C 6 H 12 O 6 ) of mass 13.2 g is placed in a test tube of radius 1.25 cm. The bottom of the test tube is a membrane that is semi-permeable to water. The tube is partially...
-
A person purchased a $181,873 home 10 years ago by paying 20% down and signing a 30-year mortgage at 8.4% compounded monthly. Interest rates have dropped and the owner wants to refinance the unpaid...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...

Study smarter with the SolutionInn App


