Use data in Table 4C.1 to calculate the entropy change for (a) The vaporization of 2.40 mol
Question:
Use data in Table 4C.1 to calculate the entropy change for
(a) The vaporization of 2.40 mol H2O(l) at 100. °C and 1 atm;
(b) The freezing of 4.50 g of ethanol, C2H5OH, at 158.7 K.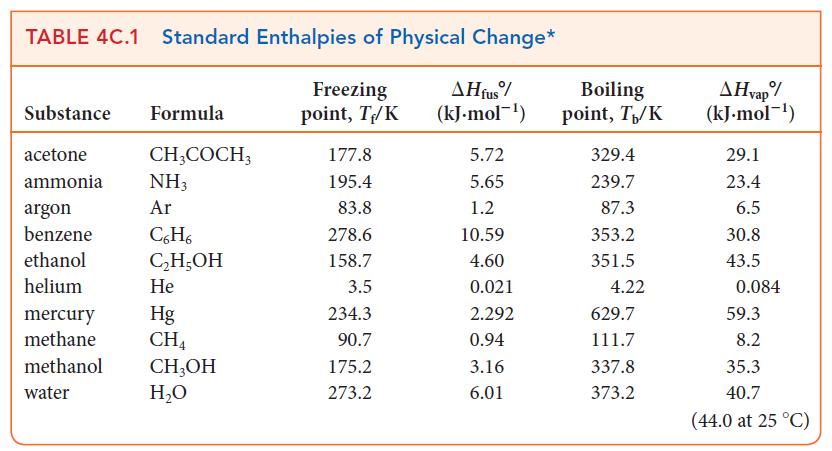
Transcribed Image Text:
TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing point, T/K Substance Formula acetone ammonia argon benzene ethanol helium CH3COCH3 NH3 Ar C6H6 C₂H5OH He mercury Hg methane CH4 methanol CH3OH water H₂O 177.8 195.4 83.8 278.6 158.7 3.5 234.3 90.7 175.2 273.2 AH fus (kJ.mol-¹) 5.72 5.65 1.2 10.59 4.60 0.021 2.292 0.94 3.16 6.01 Boiling point, T₁/K 329.4 239.7 87.3 353.2 351.5 4.22 629.7 111.7 337.8 373.2 A Hvap%/ (kJ.mol-¹) 29.1 23.4 6.5 30.8 43.5 0.084 59.3 8.2 35.3 40.7 (44.0 at 25 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
The entropy change AS for a process is given by the formula AS AH where AH is the enthalpy ...View the full answer

Answered By

Raunak Agarwal
Teaching is my hobby and now my profession. I teach students of CA and CFA(USA) in batches of 100 students and have a 5 year experience.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the entropy change for the vaporization of liquid methane and hexane using the following data: Compare the molar volume of gaseous methane at 112 K with that of gaseous hexane at 342 K. How...
-
Calculate the entropy change for a process in which 3.00 moles of liquid water at 0oC is mixed with 1.00 mole of water at 100.oC in a perfectly insulated container. (Assume that the molar heat...
-
Use data in Table 4C.1 or Appendix 2A to calculate the entropy change for (a) The freezing of 1.00 mol H 2 O(l) at 0.00C; (b) The vaporization of 50.0 g of ethanol, C 2 H 5 OH, at 351.5 K. TABLE 4C.1...
-
CMOS Chips is hedging a 20-year, $21 million, 8% bond payable with a 20-year interest rate swap and has designated the swap as a fair value hedge. The agreement called for CMOS to receive payment...
-
(a) Describe the philosophy and approach of just-in-time processing. (b) Identify the major elements of JIT processing.
-
Show that 22 (n!)? (1 - x*)" dx - (2n + 1)!
-
4. The capital accounts of the partnership of New, Sha, and Jac on June 1, 2016, are presented, along with their respective profit and loss ratios: New $139,200 1/2 Sha 208,800 1/3 Jac 96,000 1/6...
-
New lithographic equipment, acquired at a cost of $800,000 at the beginning of a fiscal year, has an estimated useful life of five years and an estimated residual value of $90,000. The manager...
-
4 2 6 Required information C4-4 (Static) From Recording Transactions (Including Adjusting Journal Entries) to Preparing Financial Statements and Closing Journal Entries (Chapters 2, 3, and 4) (LO...
-
A sample of gas in a cylinder of volume 3.42 L at 298 K and 2.57 atm expands to 7.39 L by two different pathways. Path A is an isothermal, reversible expansion. Path B has two steps. In the first...
-
In the manufacture of nitric acid by the oxidation of ammonia, the first product is nitric oxide, which is then oxidized to nitrogen dioxide. From the standard reaction enthalpies, calculate the...
-
During 2018, Roberto sold 830 shares of Casual Investor Mutual fund for $8.875 per share. The shares were purchased on the following dates: Date Shares Price May 31, 2014 400 $9.375 September 18,...
-
D manufacturing Company uses a process cost system. In the second department, Department X, spoiled units occur when units are 70% complete. Direct Materials are added at the end of the process....
-
This week, I read Pat Friman's 2021 article, "There Is no Such Thing As a Bad Boy: The Circumstances View of Problem Behavior," on adopting The Circumstances View when working with clients. In this...
-
Download the labour force statistic for Australia from the ABS website here. Use the data to answer the following questions only. You do not have to analyze the data. Show proof when you make...
-
Split Corporation manufactures products X, Y, and Z from a joint production process. Joint costs are allocated to products based on relative sales values of the products at the split-off point....
-
A television show conducted an experiment to study what happens when buttered toast is dropped on the floor. When 44 buttered slices of toast were dropped, 28 of them landed with the buttered side up...
-
Extranuclear inheritance often correlates with maternal inheritance. Even so, paternal leakage is not uncommon. What is paternal leakage? If a cross produced 200 offspring and the rate of...
-
The Ranch 888 Noodle Company sells two types of dried noodles:ramen, at $6.50 per box, and chow fun, at $7.70 per box. So farthis year, the company has sold a total of 110,096 boxes ofnoodles,...
-
Another way of producing highly crosslinked polyesters is to use glycerol. Alkyd resins are a polymer of this type. The polymer forms very tough coatings when baked onto a surface and is used in...
-
Distinguish between the primary, secondary, and tertian structures of a protein. Give examples of the types of forces that maintain each ty pe of structure.
-
Which of the amino acids in Fig. contain the following functional groups in their R group? a. Alcohol b. Carboxylic acid c. Amine d. Amide Nonpolar R groups OH OH HyC Glycine (Gly) Alanine Ala) OH ...
-
The following is part of the computer output from a regression of monthly returns on Waterworks stock against the S&P 5 0 0 index. A hedge fund manager believes that Waterworks is underpriced, with...
-
Doisneau 25-year bonds have an annual coupon interest of 8 percent, make interest payments on a semiannual basis, and have a $1,000 par value. If the bonds are trading with a market's required yield...
-
Hite corporation intends to issue $160,000 of 5% convertible bonds with a conversion price of $40 per share. The company has 40,000 shares of common stock outstanding and expects to earn $600,000...

Study smarter with the SolutionInn App


