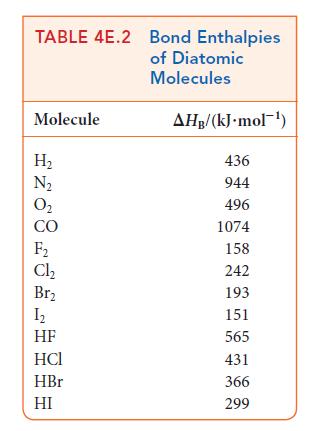
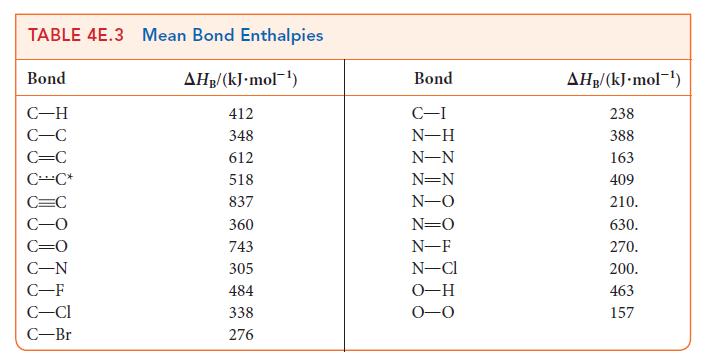
Use the bond enthalpies in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) 3
Question:
Use the bond enthalpies in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for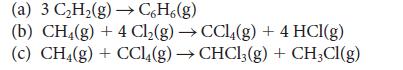
Transcribed Image Text:
(a) 3 C₂H₂(g) →CH(g) (b) CH4(g) + 4 Cl₂(g) → CCl4(g) + 4 HCl(g) (c) CH4(g) + CCl4(g) → CHCl3(g) + CH3CI(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a 597 kJ ...View the full answer

Answered By

YOGENDRA NAILWAL
As I'm a Ph.D. student, so I'm more focussed on my chemistry laboratory. I have qualified two national level exams viz, GATE, and NET JRF (Rank 68). So I'm highly qualified in chemistry subject. Also, I have two years of teaching experience in this subject, which includes college teacher as well as a personal tutor. I can assure you if you hire me on this particular subject, you are never going to regret it.
Best Regards.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Mean bond enthalpies can be used to estimate the enthalpy of reaction when precise data are not available. Estimate the enthalpy of the reaction between liquid bromine and gaseous propene to form...
-
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) N(g) + 3 F(g) 2 NF3(g) (b) CH3CHCH(g) + HO(g) CH3CH(OH)CH3(g) (c) CH4 (g) + Cl(g) CHCl(g) + HCl(g)
-
The information in Table 4D.2 must be determined from experimental data, but because some reactions cannot be carried out directly, chemists who compile these types of tables commonly use enthalpies...
-
1. What is your brand/product? 2. Who is your target segment? 3. What are their needs/wants? 4. What message do you want to deliver to them?
-
The income statement for M2 Pizza Pie Company for the current year ended June 30 and balances of selected accounts at the beginning and the end of the year are as follows: Prepare the Cash Flows from...
-
Prove Theorem 4.1. Use the fact that all P(y) = 1
-
Describe the four components of the implementation phase of the strategic marketing process.
-
On January 1, 2013, Tonge Industries had outstanding 440,000 common shares (par $l) that originally sold for $20 per share, and 4,000 shares of 10% cumulative preferred stock (par $100), convertible...
-
1. In cell D7, use the function which will calculate the payment the customer will owe each month (display the payment as a positive value). 2. Complete the two variable data table in cells F5-K20...
-
Suppose that 50.0 g of water at 20.0 8C is mixed with 65.0 g of water at 50.0C at constant atmospheric pressure in a thermally insulated vessel. Calculate S and S tot for the process.
-
In 1750, Joseph Black performed an experiment that eventually led to the discovery of enthalpies of fusion. He placed two samples of water, each of mass 150. g, at 0.00 C (one ice and one liquid) in...
-
Why is it important to first forecast the projects local currency cash flows?
-
Maintenance costs at Red Dot Manufacturing over the past six months are listed in the following table. ( Click the icon to view the maintenance costs. ) Using the high - low method, what is the total...
-
PURPOSE: Understand Markov chains as a means to model/predict probabilistic processes in a simple case. a. Toss a coin 32 times and record the outcome (as a string of H and T). b. Compute the...
-
2. 1. The following are considered Financial Statements as per the Generally Accepted Accounting Principles (GAAP), except: a.) Profit & Loss Statement b.) Cash Flow Statement c.) Statement of...
-
In what ways do you feel prepared to use your communication skill after completing the assignment? If you don't feel prepared, share why.
-
Budgeted Activity Activity Cost Activity Base Casting $238,560 Machine hours Assembly 158,620 Direct labor hours Inspecting 24,090 Number of inspections Setup 52,540 Number of setups 43,200 Number of...
-
A radioactive substance undergoes decay as follows: Time (days) Mass (g) 0 ..................... 500 1 ..................... 389 2 ..................... 303 3 ..................... 236 4...
-
Use integration by parts to evaluate the following. Check your answer by taking the derivative. x2e-xdx
-
The reaction is the last step in the commercial production of sulfuric acid. The enthalpy change for this reaction is -227 kJ. In designing a sulfuric acid plant, is it necessary to provide for...
-
The bond energy for a C-H bond is about 413 kJ/mol in CH 4 but 380 kJ/mol in CHBr 3 . Although these values are relatively close in magnitude, they are different. Explain why they are different. Does...
-
An unknown compound contains only carbon, hydrogen, and oxygen. Combustion analysis of the compound gives mass percents of 31.57% C and 5.30% H. The molar mass is determined by measuring the...
-
Problem 12.6A (Algo) Liquidation of a partnership LO P5 Kendra, Cogley, and Mel share income and loss in a 3.21 ratio (in ratio form: Kendra, 3/6: Cogley, 2/6; and Mel, 1/6), The partners have...
-
Melody Property Limited owns a right to use land together with a building from 2000 to 2046, and the carrying amount of the property was $5 million with a revaluation surplus of $2 million at the end...
-
Famas Llamas has a weighted average cost of capital of 9.1 percent. The companys cost of equity is 12.6 percent, and its cost of debt is 7.2 percent. The tax rate is 25 percent. What is the companys...

Study smarter with the SolutionInn App


