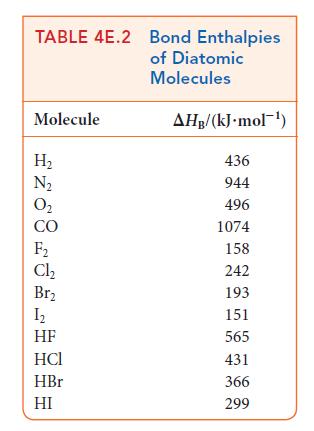
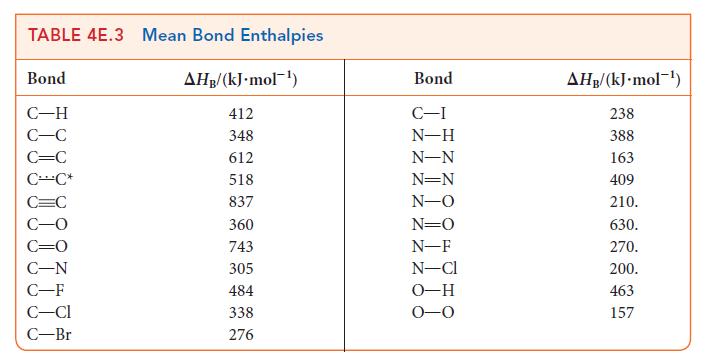
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) N(g) +
Question:
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for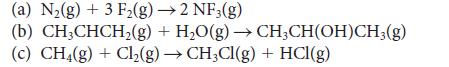
Transcribed Image Text:
(a) N₂(g) + 3 F₂(g) →2 NF3(g) (b) CH3CHCH₂(g) + H₂O(g) → CH3CH(OH)CH3(g) (c) CH4 (g) + Cl₂(g) → CH₂Cl(g) + HCl(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a 202 kJ ...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Tables 3-2 and 4-2 to estimate the DH value for a C-C bond in (a) cyclopropane: (b) cyclobutane: (c) cyclopentane; and (d) cyclohexane.
-
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) HCl(g) + F(g) HF(g) + CIF(g), given that AHB(Cl-F) = -256 kJ.mol- -1 (b) CH4(g) + HCl(g) CHCHCl(g) (c) CH4(g) + H(g)...
-
(a) Use the data in Tables 21.8 and 21.9 to calculate the relative volatility of the N 2 -O 2 system for different compositions at 65 lb /in. 2 gauge pressure. (b) How close to an ideal system is...
-
TH, has two electrons in 3) o Accodng ery, a moleule 4) o Hydrogen bondd is 2) A weak electrostatic force 4) It is not a bond 1)A weak covaient bund 3) A weak metallic force 11. Bydrogen bond may 1)...
-
During January, its first month of operations, Reyes Tool & Die accumulated the following manufacturing costs: raw materials $4,000 on account, factory labor $5,000 of which $4,200 relates to factory...
-
Determine whether the statement is true or false. If it is true, explain why. If it is false, explain why or give an example that disproves the statement. The equation y' + xy = e y is linear.
-
What distinguishes a large stock dividend from a small stock dividend? AppendixLO1
-
Currently, at a price of $1 each, 100 popsicles are sold per day in the perpetually hot town of Rostin. Consider the elasticity of supply. In the short run, a price increase from $1 to $2 is...
-
How can indirectness facilitate delivery of bad news? NEXT PAGE Select one: a. It moves the reader's attention away from the oncoming news. b. It promotes reconciliation after the news has been...
-
Oxygen difluoride is a colorless, very poisonous gas that reacts rapidly and exothermically with water vapor to produce O 2 and HF: What is the change in internal energy for the reaction of 1.00 mol...
-
Without performing any calculations, predict whether there is an increase or a decrease in entropy of the system for each of the following processes: (a) Cl 2 (g) + H 2 O(l) HCl(aq) + HClO(aq); (b)...
-
If requirements are easily understandable and defined, which of the following models is best suited? (a) Waterfall model (b) Prototyping model (c) Spiral model (d) None of these
-
4) This question concerns the simulation of price trajectories in the Black-Scholes model. We therefore want to simulate price vectors: (St St, Str); where tiit, i=0,1,..., n. The total number of...
-
Consider a pure exchange economy with two goods, (x,y), and two consumers, (1,2). Con- sumers' endowments are e (4,2) and e = (6,6) and their preferences are represented by utility functions: u(x,y)...
-
O The national highways agency releases information on the pro- portion of people not wearing seatbelts, aggregated by city. The data comes from random traffic stops conducted between 8am and 9am on...
-
Ivanka's Budgeted Income Statement You are the accountant for Ivanka Ltd which operates a small mixed business. The following estimates relate to the base year (Year 1): Sales of product A $100 000...
-
1. Implement the function of a XNOR gate by a 2 to 4 decoder. Use logic gates if needed at the output. 2. The following question is to design an octal to binary encoder. a) Write down the truth table...
-
Describe the differences among dominance, incomplete dominance, codominance, and overdominance.
-
Evenflow Power Co. is considering a new project that is a little riskier than the current operations of the company. Thus, management has decided to add an additional 1.5% to the company's overall...
-
Three gas- phase reactions were run in a constant-pressure piston apparatus as illustrated. For each reaction, give the balanced reaction and predict the sign of w (the work done) for the reaction....
-
Consider the following changes: a. N2(g) N2(l) b. CO(g) + H2O(g) H2(g) + CO2(g) c. Ca3P2(s) + 6H2O(l) 3Ca(OH)2(s) + 2PH3(g) d. 2CH3OH(l) + 3O2(g) 2CO2(g) + 4H2O(l) e. I2(s) I2(g) At constant...
-
Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 40 cm3. If the combustion of this mixture releases 950. J of energy, to what volume will the gases...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


