Use the data in Appendix 2A to write balanced equations and calculate the heat released when (a)
Question:
Use the data in Appendix 2A to write balanced equations and calculate the heat released when
(a) 1.00 mol and
(b) 1.00 g of each of the following compounds is burned in excess oxygen: propane, butane, and pentane. Is there a trend in the amount of heat released per mole of molecules or per gram of compound? If so, what is it?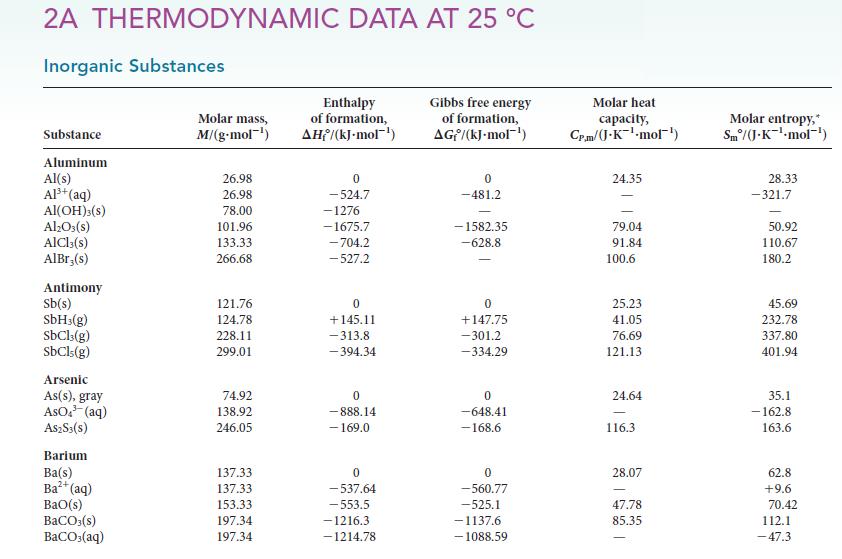
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) Al(OH)3(s) Al2O3(s) AlCl3(s) AlBr;(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray ASO. (aq) AS2S3(s) Barium Ba(s) Ba+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3. -1214.78 Gibbs free energy of formation, AG/(kJ-mol) 0 -481.2 -1582.35 -628.8 - 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cp.m/(J.K-mol) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J.K-mol-') 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 - 162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The balanced equations are C3H8g 5 Og 3 COg 4HO1 C4H0g Og 4 COg ...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In the following exercises, you will use the data in the Solmaris Condominium Group database shown in Figures 1-21 through 1-25 in Chapter 1. (If you use a computer to complete these exercises, use a...
-
In the following exercises, you will use the data in the TAL Distributors database shown in Figure 2-1 in Chapter 2. (If you use a computer to complete these exercises, use a copy of the original TAL...
-
Use the data in Appendix 3 to calculate the equilibrium constant for the reaction Agl(s) Ag+(aq) + I2(aq) at 25C. Compare your result with the Ksp value in Table 16.2.
-
A quality control manager at a manufacturing facility has taken four samples with four observations each of the diameter of a part. (a) Compute the mean of each sample. (b) Compute an estimate of the...
-
Find vo(t) for t > 0 in the network in figure and plot the response including the time interval just prior to moving theswitch. 10 kn 5 kn ww 10 kn 10 F 100 v(+ vo(1) 100 mH
-
Smithston Corporation leased equipment to Dayplanner Co. on January 1, 2011. The terms of the lease called for annual lease payments to be made at the first of each year. Smithstons implicit interest...
-
13. Working together, draw up a list of the apparent positive and negative attributes of each candidate. What can the hiring manager do to avoid making a biased choice?
-
Granite Engineering Ltd. has entered into a contract beginning January 1, 2013, to build a bridge in Tuktoyuktuk Shores. It estimates that the bridge will cost $14.8 million and will take three years...
-
AMP Corporation ( calendar - year - end ) has 2 0 2 0 taxable income of $ 1 , 9 0 0 , 0 0 0 for purposes of computing the 1 7 9 expense. During 2 0 2 0 , AMP acquired the following assets: ( Use...
-
Write the general formula of each of the following types of compounds, using R to denote an organic group: (a) Amine; (b) Alcohol; (c) Carboxylic acid; (d) Aldehyde.
-
Why do branched-chain alkanes have lower melting points and boiling points than unbranched alkanes with the same number of carbon atoms?
-
In Problems 5-15 and 5-16, three different forecasts were developed for the demand for fertilizer. These three forecasts are a 3-year moving average, a weighted moving average, and a trend line....
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
Noble gases (Group 18 in the periodic table) have the following volume concentrations in dry air: He, 5.24 ppm; Ne, 18.2 ppm; Ar, 0.934%; Kr, 1.14 ppm; Xe, 87 ppb. (a) A concentration of 5.24 ppm He...
-
What is the difference between adsorption and absorption?
-
The copper pipe has an outer diameter of 3 in. and an inner diameter of 2.5 in. If it is tightly secured to the wall at C and a uniformly distributed torque is applied to it as shown, determine the...
-
The rod has a diameter of 1 in. and a weight of 15 lb/ft. Determine the maximum torsional stress in the rod at a section located at B due to the rods weight. 4.5 ft -1.5 ft 1.5t B. 1.5 ft 4 ft
-
The rod has a diameter of 1 in. and a weight of 10 lb/ft. Determine the maximum torsional stress in the rod at a section located at A due to the rods weight. 4.5 ft 1.5 ft 1.5 ft B. 4 ft
-
Julia Co. purchased a trading debt security on October 4 of the current year for $50,000. The market value of the stock investment at year-end is $47,000. What value will be reported in net income...
-
1. (A nice inharitage) Suppose $1 were invested in 1776 at 3.3% interest compounded yearly a) Approximatelly how much would that investment be worth today: $1,000, $10,000, $100,000, or $1,000,000?...
-
Why Should not the government subsidize home buyers who make less than $120K per year. please explain this statement

Study smarter with the SolutionInn App


