Use the information in Table 5G.2 to determine the value of K at 300 K for the
Question:
Use the information in Table 5G.2 to determine the value of K at 300 K for the reaction 2 BrCl (g) + H2(g) ⇌ Br2(g) + 2 HCl(g).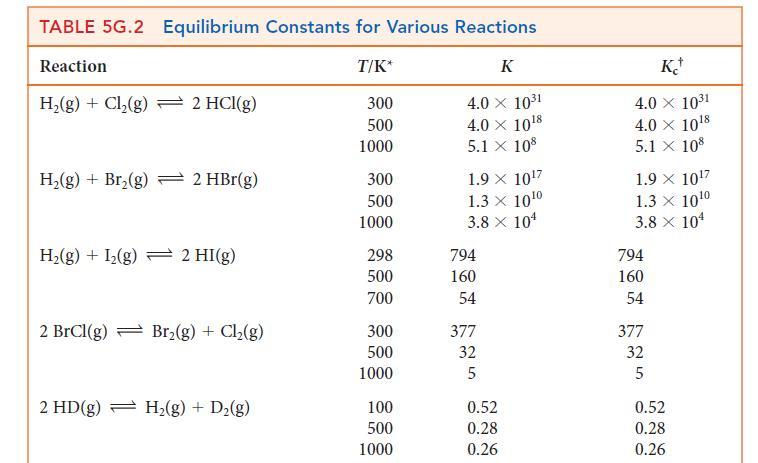
Transcribed Image Text:
TABLE 5G.2 Equilibrium Constants for Various Reactions Reaction H₂(g) + Cl₂(g) 2 HCl(g) H₂(g) + Br₂(g) → 2 HBr(g) H₂(g) + I₂(g) 2 HI(g) 2 BrCl(g) Br₂(g) + Cl₂(g) 2 HD(g) → H₂(g) + D₂(g) T/K* 300 500 1000 300 500 1000 298 500 700 300 500 1000 100 500 1000 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 × 10¹7 1.3 × 10¹0 3.8 x 10¹ 794 160 54 377 32 5 0.52 0.28 0.26 K 4.0 × 10³1 4.0 × 10¹8 5.1 X 108 1.9 X 10¹7 1.3 X 10¹⁰ 3.8 x 10¹ 794 160 54 377 32 5 0.52 0.28 0.26
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
K ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the information in Table 2.5 to predict the standard reaction enthalpy of2 H2 (g) + 02(g) 2 H2O (1) at 100C from its value at 25C.
-
(a) In an experiment, 5.0 mmol Cl 2 (g) was sealed into a reaction vessel of volume 2.0 L and heated to 1200. K, and the dissociation equilibrium was established. What is the equilibrium composition...
-
The reaction (CH3)3CBr + OH- (CH3)3COH + Br2 in a certain solvent is first order with respect to (CH3)3CBr and zero order with respect to OH-. In several experiments the rate constant k was...
-
The General Auditors Office (GAO) of ABC jurisdiction issued a report on the XYZ Electric Cooperative, a large member-owned utility. This report reviewed the work of MNO Consulting. MNO found...
-
(Multiple choice) (1) True or false: (a) The equivalent capacitance of two capacitors in parallel equals the sum of the individual capacitances. (b) The equivalent capacitance of two capacitors in...
-
The ultimate tensile strength of an AISI 1117 cold-drawn steel is Weibullian, with Su = W [70.3, 84.4, 2.01]. What are the mean, the standard deviation, and the coefficient of variation?
-
(f) What is the distinguishing feature of this family?
-
As a hedge fund specializing in locating attractively priced fixed-income instruments, Millennium Cerberus Fund is now reviewing its overall bond allocation strategy following the recent "liquidity...
-
Single Plantwide Factory Overhead Rate Salty Sensations Snacks Company manufactures three types of snack foods: tortilla chips, potato chips, and pretzels. The company has budgeted the following...
-
Amanda Autry and Carley Wilson are partners in A & W Gift Shop, which employs the individuals listed below. Paychecks are distributed every Friday to all employees. Based on the information given,...
-
What is the molality of ethylene glycol, C 2 H 6 O 2 , in an aqueous solution used for antifreeze, given that the mole fraction of ethylene glycol is 0.250?
-
Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of water when the atmospheric pressure is (a) 60. kPa; (b) 160. kPa. FIGURE 5A.3 120 100 Vapor pressure, P/k Pa 40 20 101.325...
-
If you get a fill-up of gas, you will get a free car wash. You get a fill-up of gas. Therefore, you will get a free car wash. Determine whether each argument in Problems 928 is valid or invalid. If...
-
The cable supports two cylinders as shown. Cylinders E and F have a mass of 15 kg and 35 kg, respectively. Determine the sag dc and the tension in each segment of the cable. 2 m 2.5 m -2.5m- 2 m dc E...
-
A raft foundation having dimensions of 35 m x 35 m in plan is to be constructed on a deep deposit of sand. Foundation depth and the ground water table are both 5 m below the surface. Unit weight of...
-
Determine the number of 2 X 4 @ 92 5/8" studs needed for the garage in Figures 14.63 and 14.64. The studs are spaced 16 inches on center. Add two studs for each door and corner. Ignore the gable ends...
-
Sketch a cumulative flow diagram that represents the growth and dissipation of a rush hour period at a toll bridge with time-independent capacity. 1) Identify on the diagram: the arrival curve A(t),...
-
Plot the reciprocal lattice for a polycrystalline sample o fa material with a simple tetragonal structure and lattice parameters a = 4.0 A and c = 5.0 A. (Use a two dimensional section through the...
-
a) What is the major attraction of a HIDS? b) What are the two weaknesses of host IDSs? c) List some things at which host operating system monitors look?
-
The Pletcher Transportation Company uses a responsibility reporting system to measure the performance of its three investment centers: Planes, Taxis, and Limos. Segment performance is measured using...
-
A van der Waals gas has a value of z = 1.00061 at 410. K and 1 bar and the Boyle temperature of the gas is 195 K. Because the density is low, you can calculate V m from the ideal gas law. Use this...
-
A sample of Na2SO4(s) is dissolved in 225 g of water at 298 K such that the solution is 0.325 molar in Na 2 SO 4 . A temperature rise of 0.146C is observed. The calorimeter constant is 330. J K 1 ....
-
Assign a name for each of the following compounds. a. b. c.
-
DISCUSSION ACTIVITY All jurisdictions have legislation protecting seniority and benefits for qualified employees who are members of the Canadian Forces Reserves and who are deployed for active...
-
Firm J has net income of $90,160, sales of $980,000, and average total assets of $490,000. Firm J has net income of $90,160, sales of $980,000, and average total assets of $490,000. Required:...
-
Read Chapter 5 and the Tyco case and identify some of the signals of the misuse of acquisitions or merger reserves. How could these signals have helped the users of Tyco's financial statements...

Study smarter with the SolutionInn App


