Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of benzene when the atmospheric
Question:
Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of benzene when the atmospheric pressure is
(a) 50. kPa;
(b) 80. kPa.
FIGURE 5A.3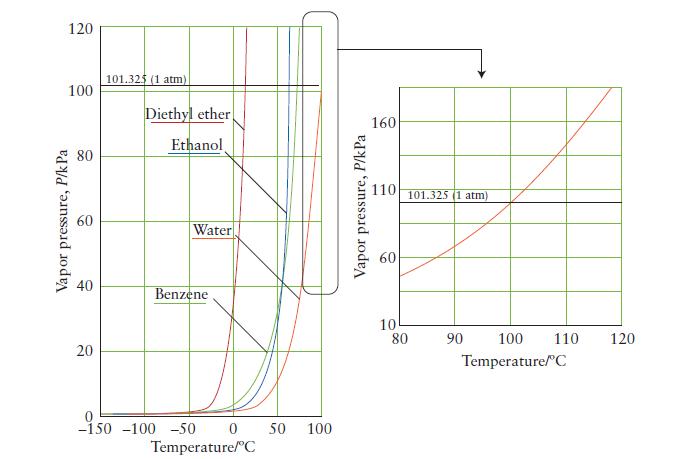
Transcribed Image Text:
120 100 Vapor pressure, P/k Pa 40 20 101.325 (1 atm) Diethyl ether Ethanol Water Benzene -1.50 -150 -100 -50 0 50 100 Temperature/C Vapor pressure, P/kPa 160 110 10 80 101.325 (1 atm) 90 100 110 Temperature/C 120
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The boiling point of a liquid is the temperature at which its vapor pressure eq...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The vapor pressure of benzene is 100.0 mmHg at 26.1C and 400.0 mmHg at 60.6C. What is the boiling point of benzene at 760.0 mmHg?
-
The boiling point of benzene is 80.1C. Estimate: (a) Its molar heat of vaporization and (b) Its vapor pressure at 74C.
-
A liquid mixture consisting of 100 kmol of 60 mol% benzene, 25 mol% toluene, and 15 mol% o-xylene is flashed at 1 atm and 100C. (a) Compute the amounts of liquid and vapor products and their...
-
In Exercises 7-9, find the indicated measure. The area of a circle is 380 square inches. Find the radius.
-
In field-flow fractionation, could a turbulent-flow field be used? Why or why not?
-
Design an infinite-life helical coil extension spring with full end loops and generous loop-bend radii for a minimum load of 9 lbf and a maximum load of 18 lbf, with an accompanying stretch of in....
-
(a) Fit the mixed model introduced in Chapter 1 to these data. Obtain the intercept and slope of the line of best fit relating log(house price) to latitude. Obtain the BLUP for the effect of each...
-
Tiny Corp. prepares monthly bank reconciliations of its checking account balance. The bank statement for October 2009 indicated the following: Balance, October 31, 2009 ................$7,920 Service...
-
Whole Fruits Market took the following actions to improve internai controis, For each of the following actions, identify the internal control principle the company followed
-
The Dapper-Dons Partnership was formed ten years ago as a general partnership to custom tailor men's clothing. Dapper-Dons is located at 123 Flamingo Drive in City, ST, 54321. Bob Dapper manages the...
-
One step in the manufacture of sulfuric acid is the formation of sulfur trioxide by the combustion of SO 2 with O 2 in the presence of a vanadium(V) oxide catalyst. Suppose you are working out how to...
-
The phase diagram for carbon, shown here, indicates the extreme conditions that are needed to form diamonds from graphite. (a) At 2000 K, what is the minimum pressure needed before graphite changes...
-
Continuation of E5-26: Determine product profitability (Learning Objective 2) Refer to your answers in E5-26. In addition to the manufacturing overhead costs, the following data are budgeted for the...
-
1.1 Indonesia is it potential as a market for Apple? 2.1 Examination of Apple's entry strategy into the international market? 2.2 Evaluation of the entry mode(s) employed by Apple and their...
-
Dynamic, a global media agency, has recently taken over MediaHype, a local agency in Melbourne, to expand its Australian operations. Jeff Tan, a Chinese national, has been appointed to head the new...
-
Linear optimization models play a crucial role in improving supply chain management efficiency, both in physical and abstract network problems. Three ways they can be applied are through optimizing...
-
When I consider optimizing the portfolio allocation for both my 403(b) and CALSTRS retirement accounts, I find it crucial to employ a well-structured model to ensure that my investments align with my...
-
How can you use your understanding of diversity to develop your relationship-building skills in your healthcare career?,Explain ways in which religion can help or hinder individuals as they build...
-
What three things should a firm do about disaster recovery planning for office PCs?
-
2. In the circuit given in Figure 2, i,(t) = 5.67cos(5t)A and v (t) = 70.71 cos(5t 60) V a) Find the equivalent load impedance. State whether the load is inductive or capacitive. b) Calculate the...
-
Provide an IUPAC name for each of the following compounds. a. b. c. d. e. I
-
Draw the structure of each of the following compounds. a. (R) -2-Ethoxy-1, 1-dimethylcyclobutane b. Cyclopropyl isopropyl ether
-
Show that the van der Waals and RedlichKwong equations of state reduce to the ideal gas law in the limit of low gas density.
-
ACC 2 0 2 Milestone One: Operational Costs Data Appendix You plan to open a small business for manufacturing pet collars, leashes, and harnesses. You have found a workshop space you can use for...
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...

Study smarter with the SolutionInn App


