Using Table 7.2, arrange the following species according to their strength as bases: H 2 O, F
Question:
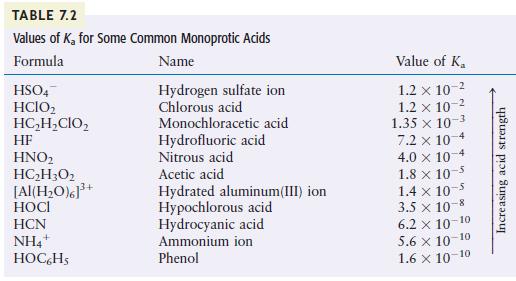
Using Table 7.2, arrange the following species according to their strength as bases: H2O, F–, Cl–, NO2–, and CN–.
Transcribed Image Text:
TABLE 7.2 Values of K for Some Common Monoprotic Acids Formula Name Hydrogen sulfate ion Chlorous acid HSO4 HCIO HCHCIO HF HNO HCH302 [Al(HO)]+ HCN NH4 HOCHS + 3+ Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of K 1.2 x 10- 1.2 x 10- 1.35 x 10-3 7.2 x 10 4.0 x 101 -4 1.8 x 10 1.4 x 10-5 3.5 10-8 6.2 10-10 5.6 10-10 1.6 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Remember that water is a stronger base than th...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(A) Refer only to the periodic table on the inside front cover, and arrange the following species in order of increasing size: Ti 2+ , V 3+ , Ca 2+ , Br - , and Sr 2+ . (B) Refer only to the periodic...
-
Compare the following two regressions: i. Y, =B + B, X, + e, ii. Y, =B + B(2X) + e, Equation i. is exactly the regression we've been working with thus far, so all the formulas we've derived thus far...
-
Arrange the following species in order of increasing oxidation number of the sulfur atom: (a) H2S, (b) S8 (c) H2SO4, (d) S2-, (e) HS-, (f) SO2, (g) SO3.
-
Besides warehouse layout decisions, what are some other applications where ranking items according to bang/buck might make sense?
-
Light with wavelength = 0.50m falls on a slit of width b = 10m at an angle 0 = 30 to its normal. Find the angular position of the first minima located on both sides of the central Fraunhofer maximum.
-
No Pairs Allowed You have a boutique that specializes in words that don't have adjacent matching characters. Bobby, a competitor, has decided to get out of the word business altogether and you have...
-
(a) Fit the model Y = ????0 + ????1X1 + ????2X2 + E to these data, and obtain estimates of ????0, ????1 and ????2.
-
Color Florists, a retail business, had the following cash receipts during January 20--. The sales tax is 5%. Jan. 1 Received payment on account from Ray Boyd, $880. 3 Received payment on account from...
-
In this lab, you will: Required: 1. Using Excels Forecast Sheet, forecast future sales for Microsoft. 2. Using Excels Forecast Sheet, forecast future income before extraordinary items for Microsoft....
-
At 60C the value of K w is 1 10 13 . a. Using Le Chteliers principle, predict whether the reaction is exothermic (releases energy) or endothermic (absorbs energy). b. Calculate [H + ] and [OH ] in...
-
A type of reaction we will study is that having a very small \(K\) value \((K < <1)\). Solving for equilibrium concentrations in an equilibrium problem usually requires many mathematical operations...
-
Suppose you wanted to form highly negative attitudes toward smoking among college students. a) Which attitude component would you focus on? Why? b) Which message characteristic would you use? Why? c)...
-
The process of translating an idea into goods and services that create value or for which clients will pay is called
-
Let f be twice differentiable with f(0) = 6, f(1) = 8, and f'(1) = 7. Evaluate the following integral. [ = 0 0 xf" (x)dx
-
Although the Chen Company's milling machine is old, it is still in relatively good working order and would last for another 10 years. It is inefficient compared to modern standards, though, and so...
-
PART-3: OFFLINE QUESTIONS - Upload files using the submission link. 1. In 2020 Starbucks began a secret project to develop a competing product against the Keurig Single Serve coffee brewer. The...
-
As a leader, what are your highest values? o What's the contribution you want to make as a leader o What makes you distinct as a leader? o Drawing from StrengthsFinder 2.0 what are your strengths as...
-
Could a revealed preference method other than travel cost have been used in the Bedford Harbor case to estimate the effects of contamination? Explain how hedonic pricing or averting behavior...
-
Why is it necessary to study the diffusion of molecules in biological systems?
-
(a) Calculate the work associated with the isothermal, reversible expansion of 1.000 mol of ideal gas molecules from 7.00 L to 15.50 L at 25.0 C. (b)Calculate the work associated with the...
-
Neutralization reactions occurring when acids and bases are mixed can be very exothermic. Suppose you are investigating how the heat released in various neutralization reactions is related to the...
-
On the basis of the structures of each of the following molecules, predict which ones would be most likely to have a residual entropy in their crystal forms at T = 0: (a) CO 2 ; (b) NO; (c) N 2 O;...
-
Each week you must submit an annotated bibliography. Entries of current events relating to the economic concepts and the impact on the company or the industry of your company. You must use acceptable...
-
Fluffy Toys Ltd produces stuffed toys and provided you with the following information for the month ended August 2020 Opening WIP Units 5,393 units Units Started and Completed 24,731 units Closing...
-
Part A Equipment 1,035,328 is incorrect Installation 44,672 is incorrect Anything boxed in red is incorrect sents 043/1 Question 9 View Policies Show Attempt History Current Attempt in Progress...

Study smarter with the SolutionInn App


