When the rate of the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g)
Question:
When the rate of the reaction 2 NO(g) + O2(g) → 2 NO2(g) was studied, the rate was found to double when the O2 concentration alone was doubled but to quadruple when the NO concentration alone was doubled. Which of the following mechanisms accounts for these observations? Explain your reasoning.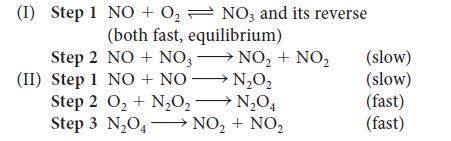
Transcribed Image Text:
(I) Step 1 NO + O₂ NO3 and its reverse (both fast, equilibrium) Step 2 NO + NO3 (II) Step 1 NO + NO NO₂ + NO₂ → N₂O₂ Step 2 O₂ + N₂O₂N₂O4 Step 3 N₂O4 NO₂ + NO₂ (slow) (slow) (fast) (fast)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Two possible mechanisms for the reaction 2 NOg O2g 2 NO2g Mechanism I Step 1NO O2 NO3 fastequilibriu...View the full answer

Answered By

Muhammed rahees M C
I'm a physics postgraduate faculty. I completed post graduation with distinction. I work as a subject matter expert in chegg in 2020.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
For each of the following seven cases, work the case twice and select the best answer. First assume that the foreign currency is the functional currency; then assume that the U.S. dollar is the...
-
The enzyme carboxypeptidases catalyses the hydrolysis of polypeptides and here we consider its inhibition. The following results were obtained when the rate of the enzymolysis of...
-
The rate of a reaction is quadrupled when the concentration of one reactant is doubled. What is the order of the reaction with respect to this reactant?
-
As Paradise Properties CEO Ajanee Kallapur began thinking about what aspects of sustainability were most important for Paradise Cove, she realized that success of the hotel was critically linked to...
-
Explain what the O_FILE_APPEND_DATA flag is used for as a value for the dwFile ACCESS argument in Windows file I/O.
-
The shock wave off the cockpit of the FA 18 in Figure has an angle of about 60o. The airplane was traveling at about 1350 km/h when the photograph was taken. Approximately what was the speed of sound...
-
P 5-5 Upstream inventory sale, 100 percent owned On February 20, 2012, Angel AG acquired all common stock of Mark AG. The book value of Mark AGs net assets was equal to fair value at the acquisition...
-
Marc Dodier is a recent university graduate and a security analyst with the Kansas City brokerage firm of Lippman, Brickbats, and Shaft. Marc has been following one of the hottest issues on Wall...
-
I am in spreadsheet modeling class and I need an idea for a nested if function for my project. The data is about NYC jobs. The variables are Job Category Career Level, Salary Range From (dependent...
-
Hrudka Corp. has manufactured a broad range of quality products since 1988. The following information is available for the company's fiscal year ended February 28, 2011. 1. The company has $4 million...
-
Ethane, C 2 H 6 , dissociates into methyl radicals by a firstorder reaction at 700C. If 820. mg of ethane is confined to a reaction vessel of volume 2.00 L and heated to 700C, what is the initial...
-
The Michaelis constant (K M ) is an index of the stability of an enzymesubstrate complex. Does a high Michaelis constant indicate a long-lived or short-lived enzymesubstrate complex? Explain your...
-
Suppose that you wish to construct a telescope that can resolve features 6.5 km across on the Moon, 384,000 km away. You have a 2.0-m-focal-length objective lens whose diameter is 11.0 cm. What...
-
Part 2 Problems 1. Jets Corp. maintains its books on a cash basis. However, the company obtained a loan of $150,000 from a local bank. The bank requires Jets Corp. to provide annual financial...
-
Company A has a well-developed brand website. They send e-mails to customers and prospects. However, the company has never used social media or mobility marketing. You have been called upon to...
-
Is Time Running Out for Bed Bath & Beyond case study and answer following questions: 3-13 analyze bed bath & beyond using the competitive forces and value chain models. 3-14 define the problem faced...
-
Marcus expresses an interest in learning more about Katie's job position, telling her that he hopes to be in the position himself one day. Katie decides to take Marcus under her wing and teach him...
-
Losing to a Weaker Foe What began as a heavily conventional military campaign to unseat the regime of Saddam Hussein had become a bitter, unconventional struggle against frustrated Sunnis who...
-
Consider the data in Exercise 8.2.9. There are two outliers among the 28 differences: the smallest value, which is 2. and the largest value, which is 18. Delete these two observations and construct a...
-
Explain why it is not wise to accept a null hypothesis.
-
Identify the hybridization state and geometry of each carbon atom in benzene. Use that information to determine the geometry of the entire molecule: .C: H. .C. Benzene
-
The following questions apply to the five compounds in Problem 5.54. (a) Which compound is meso? (b) Would an equal mixture of compounds b and c be optically active? (c) Would an equal mixture of...
-
Draw a Fischer projection for each of the following compounds, placing the CO2H group at the top. a. b. c. . .
-
Mediocre Company has sales of $120,000, fixed expenses of $24,000, and a net income of $12,000. If sales rose 10%, the new net income would be: Question 18 options: $16,800 $36,000 $13,200 $15,600
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...

Study smarter with the SolutionInn App


