Which of the following indicators in Table 6H.2 could you use for a titration of 0.20 m
Question:
Which of the following indicators in Table 6H.2 could you use for a titration of 0.20 m CH3COOH(aq) with 0.20 m NaOH(aq):
(a) Methyl orange;
(b) Litmus;
(c) Thymol blue;
(d) Phenolphthalein?
Explain your selections.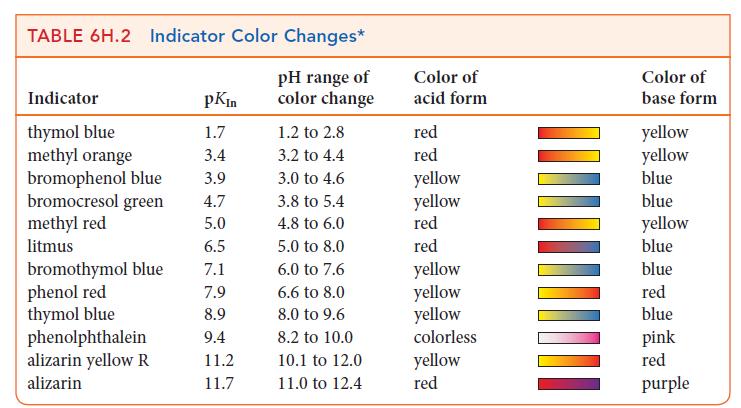
Transcribed Image Text:
TABLE 6H.2 Indicator Color Changes* pH range of color change Indicator thymol blue methyl orange bromophenol blue bromocresol green methyl red litmus bromothymol blue phenol red thymol blue phenolphthalein alizarin yellow R alizarin pKin 1.7 3.4 3.9 4.7 5.0 6.5 7.1 7.9 8.9 9.4 11.2 11.7 1.2 to 2.8 3.2 to 4.4 3.0 to 4.6 3.8 to 5.4 4.8 to 6.0 5.0 to 8.0 6.0 to 7.6 6.6 to 8.0 8.0 to 9.6 8.2 to 10.0 10.1 to 12.0 11.0 to 12.4 Color of acid form red red yellow yellow red red yellow yellow yellow colorless yellow red Color of base form yellow yellow blue blue yellow blue blue red blue pink red purple
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
d Phenolph...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Which of the following indicators in Table 6H.2 could you use for a titration of 0.20 m NH 3 (aq) with 0.20 m HCl(aq): (a) Bromocresol green; (b) Methyl red; (c) Phenol red; (d) Thymol blue? Explain...
-
You would like to invest in one of the three available investment plans: money market, bonds, or stocks. The payoffs (profits) of each plan under two possible future economic conditions, PE (poor...
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
Sunland Company has the below information for accruals for the year ended December 31st, 2022. the company adjusts its accounts annually. Chapter 4 Homework e Question 6 of 7 Current Attempt in...
-
Theory of constraints, throughput contribution, quality. Refer to the information in Exercise 19-25 in answering the following requirements. There is no connection between the requirements. 1....
-
Assume that Rayleigh's criterion gives the limit of resolution of an astronaut's eye looking down on Earth's surface from a typical space shuttle altitude of 400 km. (a) Under that that idealized...
-
A chemical product was manufactured at two-week intervals, and after a period of storage the level of available chlorine, which is known to decline with time, was measured in cartons of the product...
-
1. Whats the estimated value from the ANOVA regression given by the slopes in Table 4 for the change in sales in the Midwest if ads feature the small labor partition? Whats the simple way to get this...
-
Chris and Stefani Watanabe live with their two boys at 1400 Victoria Lane, Riverside, CA 92501. Chris is an accountant who has his own accounting practice. Stefani is an elementary school teacher....
-
Compute a depth-two decision tree for the training data in table 1 using the Gini function, C(a) = 2a(1 a) as described in class. What is the overall accuracy on the training data of the tree? XYZ...
-
The two strands of the nucleic acid DNA are held together by hydrogen bonding between four organic bases. The structure of one of these bases, cytosine, is shown below. (a) How many protons can this...
-
The two strands of the nucleic acid DNA are held together by hydrogen bonding between four organic bases. The structure of one of these bases, thymine, is shown below. (a) How many protons can this...
-
Gonzo Co. owns a building in Georgia. The building's historical cost is \($970,000,\) and \($440,000\) of accumulated depreciation has been recorded to date. During 2008, Gonzo incurred the following...
-
The following table contains the monthly operating costs of a company. Salary is not included. Determine the variance and standard deviation of the costs. Enero Febrero Marzo Abril Mayo Junio Julio...
-
Becker & Smith, CPAs, performs a financial statement review for BAM Markets ( BAM ) . Caroline, the manager on the job, learns that Don, a member of the review team, violated the independence rules....
-
Presented here are selected transactions for Sheridan Inc. during August of the current year. Sheridan uses a perpetual inventory system. It estimates a return rate of 10%, based on past experience....
-
. Complete both parts (a) and (b) below. ). In1 (a) Let X11, X12, ..., X be a random sample of size n from a population with mean and variance . Let X21, X22,..., X2n2 be a random sample of size n...
-
41. Let S be the cone z = x + y, z 2, oriented with outward unit normal. Use Stokes' theorem to evaluate the flux integral for the vector field SJ (V x F). ndS F(x, y, z) = (x y)i + 2zj + xk. -
-
6.7.15 Researchers reported the mean ± SE foot circumferences of many male and female Sumatran elephants as 125.6 ± 4.4 cm for the males and 151.7 ± 3.3 for the females.24 You...
-
Complete the following acid-base reactions: (a) HCCH + NaH
-
Predict the change in the partial pressure of CO 2 as the pressure is increased at constant temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for which H o R = 283.0 kJ mol 1 .
-
Predict the change in the partial pressure of CO 2 as the pressure is increased at constant temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for which H o R = 283.0 kJ mol 1 .
-
Predict the change in the partial pressure of CO 2 as Xe gas is introduced into the reaction vessel at constant pressure and temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for which H o R =...
-
The rate of return on Cherry Jalopies, Inc., stock over the last five years was 14 percent, 11 percent, 4 percent, 3 percent, and 7 percent. What is the geometric return for Cherry Jalopies, Inc.?
-
U.S. GAAP specifies all of the following characteristics of variable interest entities except: A. Equity holders hold less than 5% of the entitys voting stock. B. Equity holders do not have voting...
-
Rank the following three stocks by their risk-return relationship, best to worst. Night Ryder has an average return of 10 percent and standard deviation of 27 percent. The average return and standard...

Study smarter with the SolutionInn App


