Write the chemical formula for the conjugate base of formic acid, HCOOH and calculate its pK b
Question:
Write the chemical formula for the conjugate base of formic acid, HCOOH and calculate its pKb from the pKa of formic acid (see Table 6C.1).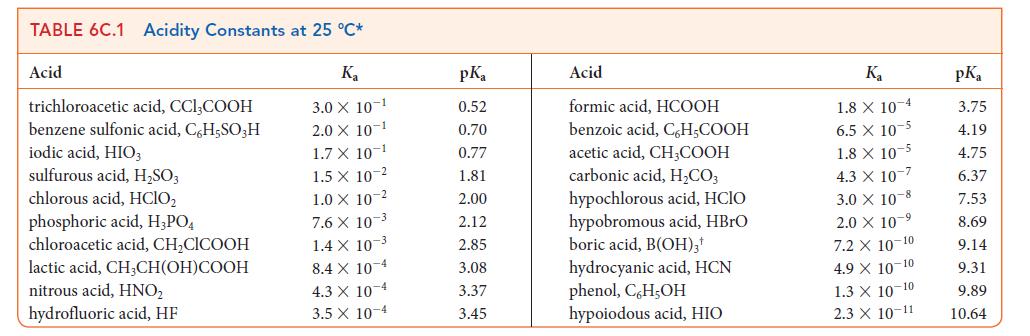
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* K₂ 3.0 X 10-1 2.0 X 10-1 1.7 X 10-1 1.5 X 1.0 X 10-2 Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C6H5SO3H iodic acid, HIO3 sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H3PO4 chloroacetic acid, CH₂CICOOH lactic acid, CH3CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF 10 2 7.6 X 10-3 1.4 X 10-3 8.4 X 10 4 4.3 X 10-4 3.5 x 10-4 pKa 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H₂COOH acetic acid, CH,COOH carbonic acid, H₂CO3 hypochlorous acid, HCIO hypobromous acid, HBrO boric acid, B(OH)3¹ hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO K₂ 1.8 X 10 4 6.5 x 10-5 1.8 X 10-5 4.3 X 107 3.0 X 108 2.0 × 10-⁹ 7.2 X 10-10 4.9 X 10-10 1.3 X 10-10 2.3 X 10-11 pKa 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
HCO pK...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
1. Does squinting help you see things more clearly? Explain. 2. Why, when there are very low-light conditions, are you unable to see color? 3. Stand in a well-lit room facing a mirror. Close your...
-
Find α and β that solve the following equation: 1 2 2 1
-
In order to have $391,185 in 26 years, how much needs to be deposited each month into a bank account whose annual rate is 1.8% with monthly compounding?
-
Standard-costing method, assigning costs. Refer to the information in Exercise 17-24. Suppose Bio Dec determines standard costs of $6.60 per equivalent unit for direct materials and $10.40 per...
-
A randomly generated data stream consists of equiprobable binary symbols 0 and 1. It is encoded into a polar nonreturn-to-zero waveform with each binary symbol being defined as follows: (a) Sketch...
-
Take the natural log of home runs, and perform a regression of ln home runs on batting average. Obtain a normal probability plot of the standardized residuals from this regression. Does the normal...
-
K&J Web Designs creates Web sites for businesses. K&J has a basic Web site creation package that it offers for a flat fee of $600.This package includes everything that a business would need to have a...
-
Your friend, Anthony, owns a food truck business that serves gourmet food to his customers in the residential suburbs of Singapore. He has prepared his accounts on a worksheet ( see Excel attachment...
-
1. Liam Richardson is the business manager for the Smith & Lyngate Insurance agencies in the state of Maryland. Liam is interested in increasing the number of agents in Baltimore and plans to buy...
-
Identify (a) The Brnsted acid and base in the following reaction, and (b) The conjugate base and acid formed: HNO3(aq) + HPO (aq) NO3 (aq) + HPO4 (aq)
-
Calcium acetate, Ca(CH 3 CO 2 ) 2 (aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they...
-
Refer to Figure 3.37 in Exercise 15 and construct a bar chart to depict the same data in a way that is fair and objective. Figure 3.37 Exercise 15 Figure 3.37 depicts the amounts of daily oil...
-
Skinovations needs to put together a Production schedule for next week and has asked its marketing team to give its forecasts for next week's sales. The team has used two different forecasting...
-
If a potential leader viewed her least preferred co-worker in favorable terms, how would Fiedler's Model describes this leader?
-
You have just been hired as a financial analyst for Lydex Company, a manufacturer of safety helmets. Your boss has asked you to perform a comprehensive analysis of the company s financial statements,...
-
For our first discussion you should locate a research article in which a quantitative study is reported. This article should not be a theoretical article or a methods article, but should describe...
-
A box is separated by a partition which divides its volume in the ration of 3:1. the larger portion of the box contains 1000 molecules of Ne gas; the smalled portion contains 100 molecules of He gas....
-
Ten patients with high blood pressure participated in a study to evaluate the effectiveness of the drug Timolol in reducing their blood pressure. The accompanying table shows systolic blood pressure...
-
On October 1, 2014, the Dow Jones Industrial Average (DJIA) opened at 17,042 points. During that day it lost 237 points. On October 2 it lost 4 points. On October 3 it gained 209 points. Deter-mine...
-
Show that the three wave functions in Equation (14.11) are normalized.
-
Discuss why a quantum mechanical particle in a box has zero point energy in terms of its wavelength.
-
How does an expectation value for an observable differ from an average of all possible eigenvalues?
-
The market price of a stock is $24.55 and it is expected to pay a dividend of $1.44 next year. The required rate of return is 11.23%. What is the expected growth rate of the dividend? Submit Answer...
-
Suppose Universal Forests current stock price is $59.00 and it is likely to pay a $0.57 dividend next year. Since analysts estimate Universal Forest will have a 13.8 percent growth rate, what is its...
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...

Study smarter with the SolutionInn App


