Write the equilibrium constant for the reaction HIO 3 (aq) + NH 2 NH 2 (aq)
Question:
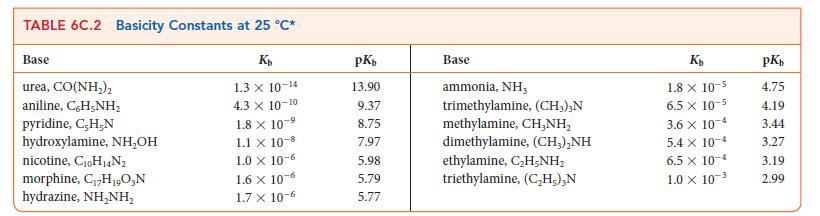
Write the equilibrium constant for the reaction HIO3(aq) + NH2NH2(aq) ⇌ NH2NH3 + (aq) + IO3– (aq) and calculate the value of K at 298 K using the data in Tables 6C.1 and 6C.2.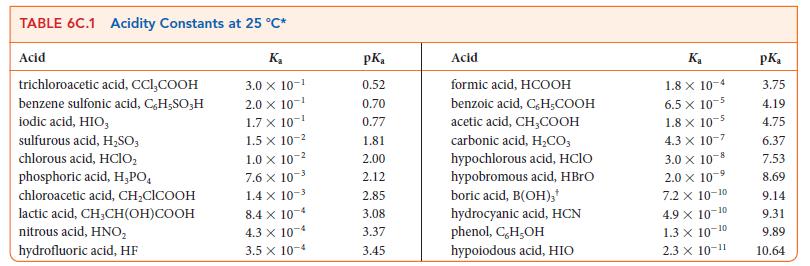 .
.
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 C* Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H-SO;H iodic acid, HIO, sulfurous acid, HSO3 chlorous acid, HClO phosphoric acid, H,PO chloroacetic acid, CHClCOOH lactic acid, CH,CH(OH)COOH nitrous acid, HNO hydrofluoric acid, HF K 3.0 X 10- 2.0 10- 1.7 X 10- 1.5 x 10- 1.0 x 10- 7.6 X 10- 1.4 x 10- 8.4 x 10-4 4.3 x 10-4 3.5 x 10-4 pK 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H-COOH acetic acid, CHCOOH carbonic acid, HCO; hypochlorous acid, HClO hypobromous acid, HBrO boric acid, B(OH)3* hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO Ka 1.8 x 10-4 6.5 x 10-5 1.8 x 10-5 4.3 X 10-7 3.0 10-8 2.0 x 10-5 -9 7.2 x 10-10 4.9 X 10- -10 1.3 10-10 2.3 10-11 pK 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
K NHNH IO KHIOxK NHNH N...View the full answer

Answered By

Hamza Amjad
Currently I am student in master degree program.from last two year I am tutring in Academy and I tought many O/A level student in home tution.
4.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Estimate the slope of the tangent line at the point indicated. y(x) = 1 x + 2 x = 2
-
Define SPARCS and discuss the following: 1. Purpose - why was it developed. 2. Review data elements collected. 3. Identify how this data is meaningful to New York State and other healthcare...
-
Find the mass of the following thin bars with the given density function. p(x) = [x if 0 x 1 x(2-x) if 1 < x 2
-
Assume that a security is selling at INR 217 and American call and American put options are available on the stock with 3 months maturity and an exercise price of INR 210. The call is selling at INR...
-
Accounting for a byproduct. Washington Oceanic Water (WOW) desalinates and bottles sea water. The desalinated water is in high demand from a large group of environmentally conscious people on the...
-
When is it preferable to use a dense index rather than a sparse index? Explain your answer.
-
Differentiate between a purchase of assets and the purchase of a controlling interest of a company in terms of accounting procedures. AppendixLO1
-
Fong Corp. reported various transactions in 20X2: a. Equipment with an original cost of $32,500 and accumulated depreciation of $26,000 was deemed unusable and was sold for $250 scrap value. b. A new...
-
Tami Tyler opened Tamis Creations, Inc., a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain on Ms....
-
The following information is available for ADT Company, which produces special-order security products and uses a job order costing system. Overhead is applied using a predetermined overhead rate of...
-
A chemist attempts to separate barium ions from lead ions by using the sulfate ion as a precipitating agent. (a) What sulfate ion concentrations are required for the precipitation of BaSO 4 and PbSO...
-
Decide whether an aqueous solution of each of the following salts has a pH equal to, greater than, or less than 7. If pH > 7 or pH < 7, write a chemical equation to justify your answer. (a) NH 4 Br;...
-
A planned reduction in organizational size is called ______.
-
the assessment include developing gantt chart, work breakdown structure and and all task 3 are related to its respective task 2. all the instructions are given in the assignment itself. Assessment...
-
Mens heights are normally distributed with mean 68.6in. and standard deviation 2.8in. Air Force Pilots The U.S. Air Force required that pilots have heights between 64 in. and 77 in. Find the...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
ACC1810 - PRINCIPLES OF FINANCIAL ACCOUNTING Project 11: Chapter 11 - Stockholders' Equity Part B: Financial Statements The accounts of Rehearsal Corporation are listed along with their adjusted...
-
Match the term to the description. Outcome evaluation Focuses on the accomplishments and impact of a service, program, or policy and its effectiveness in attaining its outcomes set prior to...
-
A zoologist measured tail length in 86 individuals, all in the one-year age group, of the deermouse Peromyscus. The mean length was 60.43 mm and the standard deviation was 3.06 mm. The table presents...
-
Find the radius of convergence of? 1.2.3 1.3.5 (2n-1) r2n+1 -1
-
Within what range can you restrict the values of P and/or T if the following information is known about sulfur? Use Figure 8.11 to answer this problem. a. Only the rhombic solid phase is observed for...
-
The normal melting point of H 2 O is 273.15 K, and H fusion = 6010 J mol -1 . Calculate the change in the normal freezing point at 100. and 500. bar compared to that at 1 bar assuming that the...
-
Carbon tetrachloride melts at 250. K. The vapor pressure of the liquid is 10,539 Pa at 290. K and 74,518 Pa at 340. K. The vapor pressure of the solid is 270. Pa at 232 K and 1092 Pa at 250. K. a....
-
can anyone help find a news article discussing a corporations use of debt financing? Often times the financial news media will report when a company chooses to issue new bonds or take new loans.
-
a canadian investor puts money into an australian investment that offers an interest rate of 5% for six minths. The australian dollar appreciates by 6% over this period of six months. What is the...
-
Los siguientes datos corresponden a las operaciones de Turk Company el ao pasado: Ventas $ 900 000 Utilidad operativa neta $ 36 000 Margen de contribucin $ 150 000 Activos operativos promedio $ 180...
Aviation Weather Services Faa Advisory Circular 00 45f 2007th Edition - ISBN: 1560277157 - Free Book

Study smarter with the SolutionInn App


