Write the half-reactions, the balanced equation for the cell reaction, and the cell diagram for each of
Question:
Write the half-reactions, the balanced equation for the cell reaction, and the cell diagram for each of the following skeletal equations: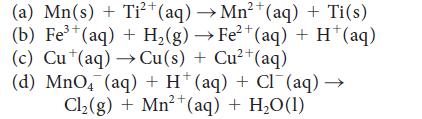
Transcribed Image Text:
2+ (a) Mn(s) + Ti²+ (aq) →→Mn²+ (aq) + Ti(s) 3+ (b) Fe³+ (aq) + H₂(g) → Fe²+ (aq) + H*(aq) (c) Cu (aq) →Cu(s) + Cu²+(aq) (d) MnO4 (aq) + H(aq) + Cl(aq) - Cl₂(g) + Mn²+ (aq) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
Here are the halfreactionsthe balanced equation for the cell reactionand the cell diagram fo...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
140+ Reviews
437+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write the half reactions for the electrolysis of the elements listed in Exercise 3.
-
The nickelcadmium cell is rechargeable. The half equations for the electrode reactions are: Cd(OH) 2 + 2e Cd + 2OH E = 0.81 V NiO 2 + 2H 2 O + 2e Ni(OH) 2 + 2OH E = +0.49 V a. Which of these...
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
Wansley Portal Inc., a large Internet service provider, is evaluating the possible acquisition of Alabama Connections Company (ACC), a regional Internet service provider. Wansley's analysts project...
-
Transferred-in costs, weighted-average method. Publish, Inc. has two departments: Printing and Binding. Each department has one direct-cost category (direct materials) and one indirect-cost category...
-
Consider a discrete memory less source whose alphabet consists of K equiprobable symbols. (a) Explain why the use of a fixed-length code for the representation of such a source is about as efficient...
-
Interpret the value of the slope b1.
-
The following information is available for Callaway Golf Company for the years 2008 and 2007. (Dollars are in thousands, except share information.) There were 73,139,000 shares outstanding at the end...
-
Describe the role of the trustee in a bankruptcy proceeding. What is the difference between a United States Trustee and a case ( private ) trustee?
-
4. (Chapter 3) Skycell, a major European cell phone manufacturer, is making production plans for the coming year. Skycell has worked with its customers (the service providers) to come up with...
-
Calculate the molar solubility in water of (a) PbBr 2 ; (b) Ag 2 CO 3 ; (c) Fe(OH) 2 .
-
Assume 20 drops per milliliter. Will a precipitate form if (a) 7 drops of 0.0029 m K 2 CO 3 (aq) are added to 25.0 mL of 0.0018 m CaCl 2 (aq); (b) 10 drops of 0.010 m Na 2 CO 3 (aq) are added to 10.0...
-
Explain the difference between directional policy matrices and multiple factor indices.
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
A veterinary anatomist investigated the spatial arrangement of the nerve cells in the intestine of a pony. He removed a block of tissue from the intestinal wall, cut the block into many equal...
-
All of the following assets can be depreciated, except: (a) A bulldozer (b) A copper mine (c) A surgical robot (d) A conveyor belt
-
Draw the structure of the compound with molecular formula C 6 H 15 N that exhibits the following 1 H NMR and 13 C NMR spectra: 3 Proton NMR 3.0 2.8 2.6 2.4 2.2 2.0 1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4...
-
Propose a mechanism for the following process: REN heat + N2 + CO2
-
Phenacetin was widely used as an analgesic before it was removed from the market in 1983 on suspicion of being a carcinogen. It was widely replaced with acetaminophen (Tylenol), which is very similar...
-
Given that rJ = 6.3%, rRF = 4.1%, and rM = 9.4%, determine the beta coefficient for Stock J that is consistent with equilibrium.
-
Simon Companys year-end balance sheets follow. At December 31 2017 2016 2015 Assets Cash $ 33,019 $ 37,839 $ 38,623 Accounts receivable, net 93,822 65,556 54,152 Merchandise inventory 117,963 89,253...
-
PLEASE REFER TO THE 2018 ANNUAL REPORT OF STARBUKS FOR THE YEAR FISCAL YR 2018, ENDING SEPTEMBER 30, 2018. Refer to the management discussion & analysis section and write a one page summary...

Study smarter with the SolutionInn App


