Draw the structure of the compound with molecular formula C 6 H 15 N that exhibits the
Question:
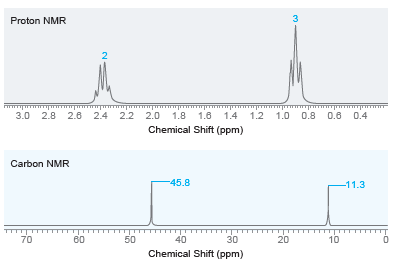
Transcribed Image Text:
3 Proton NMR 3.0 2.8 2.6 2.4 2.2 2.0 1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 Chemical Shift (ppm) Carbon NMR -45.8 -11.3 40 10 50 70 80 30 20 Chemical Shift (ppm) 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)

Answered By

Allan Simiyu
I am an adroit Writer. I am a dedicated writer having worked as a writer for 3 years now. With this, I am sure to ace in the field by helping students break down abstract concepts into simpler ideas.
5.00+
8+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the structure of the compound with molecular formula C8H11N that exhibits the following 1 H NMR and 13 C NMR spectra: Proton NMR 2 22 Chemical Shift (ppm) Carbon NMR 128.8 128.4 40.0 -126.1...
-
Deduce the structure of a compound with molecular formula C 6 H 14 O 2 that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. 100 80 60 40 20 3000 2500 Wavenumber (cm-1) 4000 3500 2000 1500...
-
Deduce the structure of a compound with molecular formula C 6 H 14 O 2 that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. 100 80 60 40 20 4000 3500 3000 2500 2000 1500 1000 Wavenumber...
-
Perhaps more surprising to Mr. Pitkin was a proposal by the VP of Marketing to make a major investment in market share by increasing promotional expenditures by $2.5 million during 1998-2000. Sales...
-
Without calculating, determine whether the value of nPr is greater than the value of nCr for the values of n and r given in the table. Complete the table using yes (Y) or no (N). Is the value of nPr...
-
By 2019, nearly $1 out of every $5 spent in the U.S. economy is projected to go for health care. The bar graph shows the percentage of the U.S. gross domestic product (GDP) going toward health care...
-
What are organization expenses? Provide examples. AppendixLO1
-
An analysis of comparative balance sheets, the current years income statement, and the general ledger accounts of Coffee Table Corp. uncovered the following items. Assume all items involve cash...
-
Smith Corporation issued 20-year, noncallable, 7.5% annual coupon bonds at their par value of $1,000 two years ago. Today, the market interest rate on these bonds is 5.6%. What is the current price...
-
1. How comparable are the two different methods? In what ways are they similar? In what ways are they different? 2. What are the positive and negative aspects of each approach that Shocker should...
-
Using benzene as your only source of carbon atoms and ammonia as your only source of nitrogen atoms, propose a synthesis for the following compound: HN NH
-
Propose a mechanism for the following process: REN heat + N2 + CO2
-
Air at 20?C flows at 1 m/s between two parallel flat plates spaced 5 cm apart. Estimate the distance from the entrance where the hydrodynamic boundary layers meet.GIVENAir flows between two parallel...
-
As an official sponsor of the Olympics, what specific benefit did John Hancock use to help drive sales in their national offices?
-
assumes that Nia has both a discount rate of zero and faces an interest rate of zero. These assumptions made calculating her constant level of consumption expenditure of $56,000 fairly...
-
Paul Petersen lives in Northern California. He owns a BMW car worth about $20,000. He wants to take a trip to Nevada with his girlfriend Patricia, who lives in Los Angeles. He takes his car into...
-
Do you see gendered patterns of interaction in personal relationships? Does knowing about gender linked patterns affect how other interpret on what happens in a relationships?
-
Significance For bone density scores that are normally distributed with a mean of 0 and a standard deviation of 1, find the percentage of scores that are significantly high (or at least 2 standard...
-
Use the graph of f to determine whether each statement in Exercises 122125 is true or false. f (-1) - f (4) = 2 -5-4-3-2. 32 y 2 3 +2+ Graph of f H HTT HE X
-
What is the maximum volume of 0.25 M sodium hypochlorite solution (NaOCl, laundry bleach) that can be prepared by dilution of 1.00 L of 0.80 M NaOCl?
-
(a) Write the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethane. (b) Explain why free-radical halogenations usually gives mixtures of products. (c) How could...
-
Draw resonance forms to show how the BHA radical is stabilized by delocalization of the radical electron over other atoms in the molecule.
-
The triphenylmethyl cation is so stable that some of its salts can be stored for months. Explain why this cation is so stable. triphenylmethyl cation
-
A company manufactures lawnmowers. Compute the total amount of period costs from thr following costs.
-
TestAnswerSavedHelp opens in a new windowSave & ExitSubmit Item 1 7 1 0 points Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1 : 2 0 : 1 8 Item 1 7 Time Remaining 1 hour 2 0 minutes 1 8 seconds 0 1...
-
Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.] Lansing Company's current-year income statement and selected balance...

Study smarter with the SolutionInn App


