You are a chemical engineer at a processing plant for soft drinks. You have been asked to
Question:
You are a chemical engineer at a processing plant for soft drinks. You have been asked to determine whether a particular solution will have a significantly different vapor pressure from pure water. Calculate the vapor pressure of water at 20°C in a solution prepared by dissolving 10.00 g of sucrose, C12H22O11, in 100.0 g of water.
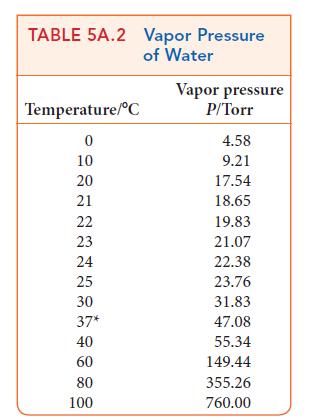
ANTICIPATE Expect a lower vapor pressure when the solute is present. However, the vapor pressure is not lowered very much by a solute, so you should expect a value slightly below that of pure water, which Table 5A.2 gives as 17.54 Torr at 20°C.
PLAN Calculate the mole fraction of the solvent (water) in the solution and then apply Raoult’s law. To use Raoult’s law, you need to know the vapor pressure of the pure solvent at the temperature of interest (Table 5A.1 or 5A.2).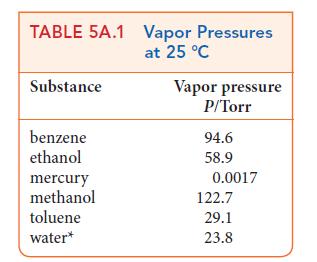
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





