Question:
You are working in the emergency room of a hospital where a patient suffering from influenza has developed metabolic alkalosis, a condition in which the pH of the blood is too high. You have available a stock solution of ammonium chloride, which is used to lower the pH of the blood of patients suffering from alkalosis, but you need to know its pH. Estimate the pH of 0.15 m NH4Cl(aq) at 25°C.
ANTICIPATE Because NH4+ is a weak acid and Cl– is neutral, you should expect pH
PLAN Treat the solution as that of a weak acid, using an equilibrium table as in Toolbox 6D.1 to calculate the composition and hence the pH. First, write the chemical equation for proton transfer to water and the expression for Ka. Obtain the value of Ka from Kb for the conjugate base by using Eq. 4a in Topic 6C (Ka = Kw/Kb). The initial concentration of the acidic cation is equal to the concentration of the cation that the salt would produce if it retained all its acidic protons.
What should you assume? Assume that (1) the extent of deprotonation is so small that the change in concentration of NH4+ is insignificant, and (2) the autoprotolysis of water does not affect the pH significantly. Check these assumptions at the end of the calculation.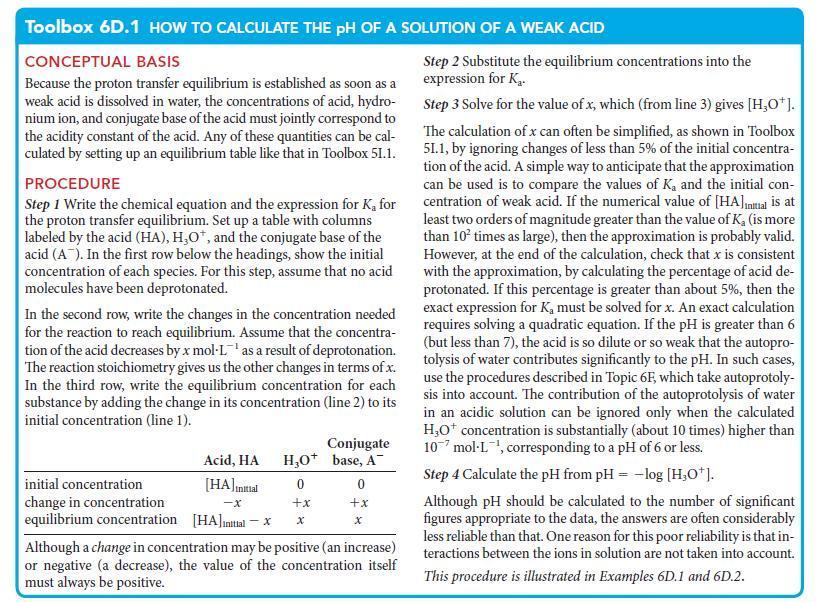
Transcribed Image Text:
Toolbox 6D.1 HOW TO CALCULATE THE PH OF A SOLUTION OF A WEAK ACID
CONCEPTUAL BASIS
Because the proton transfer equilibrium is established as soon as a
weak acid is dissolved in water, the concentrations of acid, hydro-
nium ion, and conjugate base of the acid must jointly correspond to
the acidity constant of the acid. Any of these quantities can be cal-
culated by setting up an equilibrium table like that in Toolbox 51.1.
PROCEDURE
Step 1 Write the chemical equation and the expression for K₂ for
the proton transfer equilibrium. Set up a table with columns
labeled by the acid (HA), H₂O*, and the conjugate base of the
acid (A). In the first row below the headings, show the initial
concentration of each species. For this step, assume that no acid
molecules have been deprotonated.
In the second row, write the changes in the concentration needed
for the reaction to reach equilibrium. Assume that the concentra-
tion of the acid decreases by x mol-Las a result of deprotonation.
The reaction stoichiometry gives us the other changes in terms of x.
In the third row, write the equilibrium concentration for each
substance by adding the change in its concentration (line 2) to its
initial concentration (line 1).
initial concentration
change in concentration
equilibrium concentration
Acid, HA
[HA] Initial
-X
[HA]initial-
H₂O+
0
+x
x
Conjugate
base, A™
0
+x
X
Although a change in concentration may be positive (an increase)
or negative (a decrease), the value of the concentration itself
must always be positive.
Step 2 Substitute the equilibrium concentrations into the
expression for K₂.
Step 3 Solve for the value of x, which (from line 3) gives [H₂O*].
The calculation of x can often be simplified, as shown in Toolbox
51.1, by ignoring changes of less than 5% of the initial concentra-
tion of the acid. A simple way to anticipate that the approximation
can be used is to compare the values of K, and the initial con-
centration of weak acid. If the numerical value of [HA] initial is at
least two orders of magnitude greater than the value of K₂ (is more
than 10² times as large), then the approximation is probably valid.
However, at the end of the calculation, check that x is consistent
with the approximation, by calculating the percentage of acid de-
protonated. If this percentage is greater than about 5%, then the
exact expression for K, must be solved for x. An exact calculation
requires solving a quadratic equation. If the pH is greater than 6
(but less than 7), the acid is so dilute or so weak that the autopro-
tolysis of water contributes significantly to the pH. In such cases,
use the procedures described in Topic 6F, which take autoprotoly-
sis into account. The contribution of the autoprotolysis of water
in an acidic solution can be ignored only when the calculated
H₂O* concentration is substantially (about 10 times) higher than
10 mol-L, corresponding to a pH of 6 or less.
Step 4 Calculate the pH from pH = -log [H₂O*].
Although pH should be calculated to the number of significant
figures appropriate to the data, the answers are often considerably
less reliable than that. One reason for this poor reliability is that in-
teractions between the ions in solution are not taken into account.
This procedure is illustrated in Examples 6D.1 and 6D.2.







