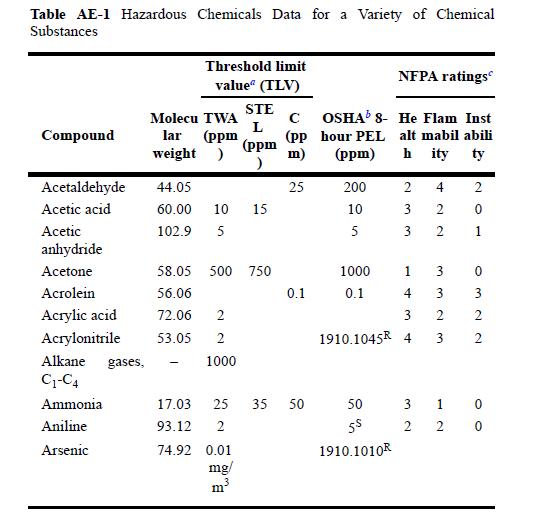
Use Appendix E to determine the hazardous properties of ammoniathat is, TLV-TWA, TLV-STEL, TLV-C, and PEL. Appendix
Question:
Use Appendix E to determine the hazardous properties of ammonia—that is, TLV-TWA, TLV-STEL, TLV-C, and PEL.
Appendix E,
Transcribed Image Text:
Table AE-1 Hazardous Chemicals Data for a Variety of Chemical Substances Compound Acetaldehyde Acetic acid Acetic anhydride Acetone Acrolein Acrylic acid Acrylonitrile Alkane gases, C-C4 Ammonia Aniline Arsenic Threshold limit value" (TLV) Molecu TWA lar (ppm weight ) STE L (ppm 44.05 60.00 10 15 102.9 5 58.05 500 750 56.06 72.06 2 53.05 2 1000 74.92 0.01 mg/ m (pp m) 25 0.1 17.03 25 35 50 93.12 2 NFPA ratings OSHA 8- He Flam Inst hour PEL alt mabil abili (ppm) hity ty 200 10 5 1000 0.1 2 4 3 2 3 2 1 4 3 1910.1045 4 50 55 1910.1010R 3 3 2 3 3 1 2 2 2 0 H 0 322 0 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Answer Sure here is the information about Ammonia from Appendix E Table AE1 Hazardous Ch...View the full answer

Answered By

Mustafa olang
Please accept my enthusiastic application to solutionInn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group. For example, I created songs to teach my three-year-old campers the camp rules, but I gave my college student daily quizzes to help her prepare for exams.
I am passionate about helping students improve in all academic subjects. I still remember my excitement when my calculus student received her first “A” on a quiz! I am confident that my passion and experience are the qualities you are looking for at solutionInn. Thank you so much for your time and consideration.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:
Students also viewed these Engineering questions
-
If the polynomial x 4 + 2x 3 + 8x 2 + 12x + 18 is divided by another polynomial x 2 + 5, the remainder comes out to be px + q. Find the values of p and q.
-
Using TLV data from Appendix E, convert TLV in ppm to mg/m3 for the following compounds. Assume a temperature of 25C and a pressure of 1 atm. a. benzene b. chlorine c. cyclohexanol d. ethylene oxide...
-
You are asked to evaluate employees' exposures to methyl n-amyl ketone during a painting operation using NIOSH Method 2553 for the sampling. You can access the method by clicking the link below: ...
-
There are two major producers of corncob pipes in the world. Suppose that the inverse demand function for corncob pipes is described by p = 120 - 4g where g is total industry output and suppose that...
-
Assuming that the pressure between the surfaces of contact of the conical bearing shown is uniform, show that the magnitude M of the couple required to overcome frictional resistance is 2 H,P R- R 3...
-
Your client, a physician, recently purchased a yacht on which he flies a pennant with a medical emblem on it. He recently informed you that he purchased the yacht and flies the pennant to advertise...
-
Narcissism in accounting students. Narcissism is a personality trait that can motivate and allow unethical behavior. Do accounting majors exhibit any more or less narcissism than any other group of...
-
A Bode diagram for a process, valve, and sensor is shown in figure.(a) Determine an approximate transfer function for this system.(b) Suppose that a proportional controller is used and that a value...
-
Given the information below, what is an estimate of this company's cost of common equity? 7-year Treasury note = 3.2% current stock price = $95.95 average annual return on S&P 500 over past five...
-
At what overpressure (Pa and psi) would 50% of structures be damaged?
-
The peak overpressure expected as a result of the explosion of a tank in a plant facility is approximated by the equation: where P is the overpressure in N/m 2 and r is the distance from the blast in...
-
Which resource access protocols prevent deadlocks caused by exclusive access to resources?
-
The English statistician Karl Pearson (1857-1936) introduced a formula for the skewness of a distribution. P = 3 ( x median ) s Pearson's index of skewness Most distributions have an index of...
-
You are to specify an orifice meter for measuring the flow rate of a $35^{\circ} \mathrm{API}$ distillate $(\mathrm{SG}=0.85$ ) flowing in a $2 \mathrm{in}$. sch 160 pipe at $70^{\circ} \mathrm{F}$....
-
Let $\theta$ and $\phi$ be the polar coordinates. Introduce the complex numbers $z$ and $\bar{z}$, where $$\begin{equation*} z=e^{i \phi} \tan (\theta / 2) \equiv \xi+i \eta \tag{5.393}...
-
Suppose the profit \(P\) (in dollars) of a certain item is given by \(P=1.25 x-850\), where \(x\) is the number of items sold. a. Graph this profit relationship. b. Interpret the value of \(P\) when...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located outside the focal length of a diverging lens. (b) Is the image real or virtual? (c) Is it upright or inverted?...
-
The drug Ziac is used to treat hypertension. In a clinical test, 3.2% of 221 Ziac users experienced dizziness (based on data from Lederle Laboratories). a. Construct a 99% confidence interval...
-
In the synthesis of the keto acid just given, the dicarboxylic acid decarboxylates in a specific way; it gives Explain. HO rather than HO
-
Describe how you would prepare approximately 2 L of 0.050 0 m boric acid, B(OH) 3 .
-
What is the true mass of water if the measured mass in the atmosphere is 5.397 4 g? When you look up the density of water, assume that the lab temperature is (a) 15C (b) 25C. Take the density of air...
-
A sample of ferric oxide (Fe 2 O 3 , density = 5.24 g/mL) obtained from ignition of a gravimetric precipitate weighed 0.296 1 g in the atmosphere. What is the true mass in vacuum?
-
At a 3% (EAR) rate of interest, you will quadruple (increase four folds) your money in approximately ____ years.
-
Smile Company makes baked goods. The budgeted sales are $620,000, budgeted variable costs are $260,400, and budgeted fixed costs are $237,800. What is the budgeted operating income?
-
Analysis of a replacement project At times firms will need to decide if they want to continue to use their current equipment or replace the equipment with newer equipment. In this case, the company...

Study smarter with the SolutionInn App


