A certain reaction has a rate given by If the concentration is to be expressed in mollliter
Question:
A certain reaction has a rate given by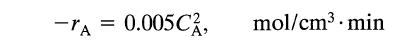
If the concentration is to be expressed in mollliter and time in hours, what would be the value and units of the rate constant?
Transcribed Image Text:
-TA = 0.005 C, mol/cm³. min
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To express the rate constant with concentration in molliter and ...View the full answer

Answered By

Antony Sang
I am a research and academic writer whose work is outstanding. I always have my customer's interests at heart. Time is an important factor in our day to day life so I am always time conscious. Plagiarism has never been my thing whatsoever. I give best Research Papers, Computer science and IT papers, Lab reports, Law, programming, Term papers, English and literature, History, Math, Accounting, Business Studies, Finance, Economics, Business Management, Chemistry, Biology, Physics, Anthropology, Sociology, Psychology, Nutrition, Creative Writing, Health Care, Nursing, and Articles.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
A certain reaction has the following general form: aA bB At a particular temperature and [A] 0 = 2.00 10-2 M, concentration versus time data were collected for this re-action, and a plot of ln[A]...
-
A certain reaction has the following general form: aA bB At a particular temperature and [A] 0 = 2.80 10-3 M, concentration versus time data were collected for this re-action, and a plot of 1/[A]...
-
Is it necessary for measurements of reaction velocity to be expressed in units of concentration per time (M s-1, for example) in order to calculate an enzyme's KM?
-
2) WWW.myitlab.com is an example of a(n). O domain name O protocol prefix OURL omni box
-
On the first day of its fiscal year, Monarch Company issued $8,000,000 of five-year, 8% bonds to finance its operations of producing and selling home electronics equipment. Interest is payable...
-
McMichael Publishers Inc. collects 75% of its sales on account in the month of the sale and 25% in the month following the sale. If sales on account are budgeted to be $302,000 for April and $263,000...
-
Research Problem 4. Find a website, other than the IRS website, that discusses the taxation of short sales of securities. Research Problem 5. Perform a Google search to find information about capital...
-
Funz Company, which produces wooden toys, is about to adopt a lean operating environment. In anticipation of the change, Letty Hernandez, Funz's controller, prepared the following list of costs for...
-
Ruiz Co. provides the following sales forecast for the next four months. 0.71 points Sales (units) April 600 May 680 June 630 July 720 eBook The company wants to end each month with ending finished...
-
On doubling the concentration of reactant, the rate of reaction triples. Find the reaction order.
-
For the complex reaction with stoichiometry A + 3B 2R + S and with second-order rate expression are the reaction rates related as follows: r A , = r B , = r R ,? If the rates are not so related,...
-
On December 31, 2009, the inventory of Powhattan Company amounts to $800,000. During 2010, the company decides to use the dollar-value LIFO method of costing inventories. On December 31, 2010, the...
-
You will need isometric dot paper for this question. Part of a pattern using four rhombuses is drawn on isometric dot paper below. By drawing two more rhombuses, complete the pattern so that it has a...
-
Fred Flores operates a golf driving range. For each of the following financial items related to his business, indicate the financial statement (or statements) in which the item would be reported:...
-
Sketch plane / intersecting plane K. Then draw a line & in plane J that intersects plane Kat a single point. A X C B D E
-
Use a graphing utility to verify any five of the graphs that you drew by hand in Exercises 126. Data from exercise 1-26 1. x + 2y = 8 3. x2y> 10 2. 3x6y 12 4. 2xy > 4
-
The following information pertains to Porter Company for 2011. Ending inventory consisted of 30 units. Porter sold 320 units at \(\$ 30\) each. All purchases and sales were made with cash. Required...
-
Old South Company purchased investments for $55,000 and plant assets for $147,000 during the current year, during which it also sold plant assets for $66,000, at a gain of $6,000. The company also...
-
If a process has a six-sigma capability, what is the process capability index? a. 1 b. 2 c. 6 d. 12
-
Tetrahydrofuran (THF) can be formed by treating 1, 4-butanediol with sulfuric acid. Propose a mechanism for this transformation. H,SO, H;SO. 1,4-Butanediol Tetrahydrofuran (THF)
-
When ethylene glycol is treated with sulfuric acid, 1, 4-dioxane is obtained. Propose a mechanism for this transformation: H,SO, Ethylene glycol 1,4-Dioxane
-
The Williamson ether synthesis cannot be used to prepare tert-butyl phenyl ether. a. Explain why this method cannot be used in this case. b. Suggest an alternative method for preparing tert-butyl...
-
Below are the revenues and cost data from King Clothes Sales Revenue $550.000 Cost of Goods Sold (40% of sales revenue) 185000 Gross Profit 365000 Operating Costs:(?) Salaries fixed 150000 Sales...
-
Land $ 33,000 16,000 Notes Payable Property Tax Expense Dividends Rent Expense Salaries Expense Salaries Payable Service Revenue Office Supplies Retained Earnings, Dec. 31, 2017 900 8,000 38,000...
-
Question 14 3 pts When a company declares a dividend, how does that transaction affect net income? No effect on net income Increases net income Decreases net income

Study smarter with the SolutionInn App


