Question: A column packed with 5-cm polypropylene saddles (a = 55 m 2 /m 3 ) is being designed for the removal of chlorine from a
A column packed with 5-cm polypropylene saddles (a = 55 m2/m3) is being designed for the removal of chlorine from a gas stream (G = 100 mol/s · m2, 2.36% Cl2) by countercurrent contact with an NaOH solution (L = 250 mol/s · m2, 10% NaOH, CB = 2736 mol/m3) at about 40-45°C and 1 atm. How high should the tower be for 99% removal of chlorine? Double the calculated height to take care of deviations from plug flow.
Data
The reaction Cl2 + 2NaOH → product is very very fast and irreversible. For these very high flow rates (close to the limits allowed) an extrapolation of the correlations in Perry 6th ed., section 14, gives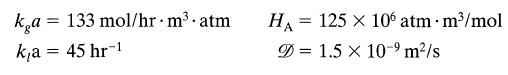
ka 133 mol/hr.m.atm ka = 45 hr-1 = HA 125 x 106 atm. m/mol = = 1.5 x 10- m/s
Step by Step Solution
3.38 Rating (148 Votes )
There are 3 Steps involved in it
The column height can be calculated using the following equation Z HAG KLa 1 ln1 X ... View full answer

Get step-by-step solutions from verified subject matter experts


