Question: At high pressure CO 2 is absorbed into a solution of NaOH in a packed column. The reaction is as follows: Find the rate of
At high pressure CO2 is absorbed into a solution of NaOH in a packed column. The reaction is as follows: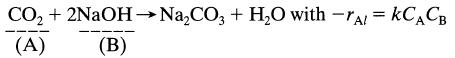
Find the rate of absorption, the controlling resistance, and what is happening in the liquid film, at a point in the column where pA = 105 Pa and CB = 500 mol/m3.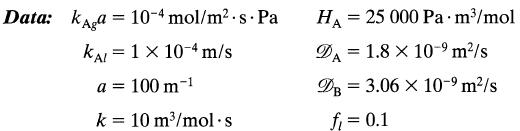
This problem was adapted from Danckwerts (1970).
CO + 2NaOH NaCO3 + HO with -TAL = KCACB (A) (B)
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


