At room temperature sucrose is hydrolyzed by the catalytic action of the enzyme sucrase as follows: Starting
Question:
At room temperature sucrose is hydrolyzed by the catalytic action of the enzyme sucrase as follows: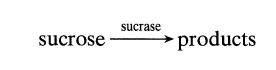
Starting with a sucrose concentration CA0 = 1.0 millimol/liter and an enzyme concentration CEO = 0.01 millimol/liter, the following kinetic data are obtained in a batch reactor (concentrations calculated from optical rotation measurements):

Determine whether these data can be reasonably fitted by a kinetic equation of the Michaelis-Menten type, or
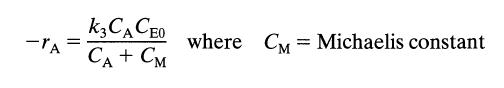
If the fit is reasonable, evaluate the constants k3 and CM. Solve by the integral method.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





