Find the overall order of the irreversible reaction from the following constant-volume data using equimolar amounts of
Question:
Find the overall order of the irreversible reaction
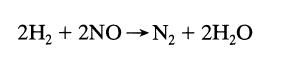 from the following constant-volume data using equimolar amounts of hydrogen and nitric oxide:
from the following constant-volume data using equimolar amounts of hydrogen and nitric oxide:
Transcribed Image Text:
2H₂ + 2NO N₂ + 2H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To find the overall order of the reaction we can use the following equation rate kHnNOm where k is t...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
The following data where measured for the reaction BF3(g) + NH3(g) -- F3BNH3(g): (a) What is the rate law for the reaction? (b)What is the overall order of the reaction? (c) Calculate the rate...
-
1. Hydrogen peroxide reacts with thiosulfate ion in slightlyacidic solution as follows: H 2 O 2 + 2S 2 O 3 2? + 2 H + ? 2H 2 O + S 4 O 6 2? The reaction rate is independent of the hydrogen...
-
Can you say GDP is underestimated or overestimated using the Expenditures approach to calculating GDP? Why?
-
What does it mean that flame is non-luminous it is yellow it is invisible It has a very bright blue inner cone it is dark
-
A hedger takes a short position in five T-bill futures contracts at the price of 98 5/32. Each contract is for $100,000 principal. When the position is closed, the price is 95 12/32. What is the gain...
-
In which situation will variable costing typically produce a higher value for net income than absorption costing? a) When units produced are greater than units sold. b) When units sold are greater...
-
17. LO.1 Faith Godwin is a dealer in securities. She has spotted a fast-rising company and would like to buy and hold its stock for investment. The stock is currently selling for $2 per share, and...
-
Best Bathware Company manufactures faucets in a small manufacturing facility. The faucets are made from zinc. Manufacturing has 50 employees. Each employee presently provides 36 hours of labor per...
-
REQUIRED] Chapter 22 Homework Flexible budget for selling and administrative expenses for a service company Digital Solutions Inc. uses flexible budgets that are based on the following data: Sales...
-
Aqueous A reacts to form R (A R) and in the first minute in a batch reactor its concentration drops from C A0 = 2.03 mol/liter to C Af = 1.97 mol/liter. Find the rate equation for the reaction if...
-
Snake-Eyes Magoo is a man of habit. For instance, his Friday evenings are all alike-into the joint with his week's salary of $180, steady gambling at "2-up" for two hours, then home to his family...
-
The Abrams, Bartle, and Creighton partnership began the process of liquidation with the following balance sheet: Cash $16,000 Liabilities $150,000 Non Cash items 434,000 Abrams, Capital 80,000...
-
Requirement 1. Prepare a horizontal analysis of the comparative income statement of McCormick Designs, Inc. Round percentage changes to one decimal place. (Round the percentages to one decimal place,...
-
GAAP looks to compare entities with an apples-to-apples valuation so that the entities can be viewed by interested parties to compare financial values and how effectively and efficiently they use...
-
Prepare a statement of cash flows for Wu using the indirect method.
-
A plate of steel with a central through-thickness flaw of length 16 mm is subjected to a stress of 350 MPa normal to the crack plane. If the yield strength of the material is 1400 MPa what is the...
-
More info The company manufactures a variety of engines for use in farm equipment. At the beginning of the current year, Dansville estimated that its overhead for the coming year would be $300,000....
-
Early in 2013, Feller Corporation was formed with authorization to issue 50,000 shares of $1 par value common stock. All shares were issued at a price of $8 per share. The corporation reported net...
-
A Bloomberg Businessweek subscriber study asked, In the past 12 months, when traveling for business, what type of airline ticket did you purchase most often? A second question asked if the type of...
-
Describe how would your reactor volume and number of reactors change if you only needed 50% conversion to produce the 200 million pounds per year required?
-
If it takes 11 minutes to cook spaghetti in Ann Arbor, Michigan, and 14 minutes in Boulder, Colorado, how long would it take in Cuzco, Peru? Discuss ways to make the spaghetti more tasty. If you...
-
How do the steps in the design of a CSTR differ from those of a CSTR or a PFR with pressure drop?
-
gnment CALCULATOR FLEEN PRINTER VERSION BACK NEXT Multiple Choice Question 142 During January 2017, its or month of operation, Osborn Enterprises earned net income of 6800 and paid dividends to the...
-
Package Corporation acquired 90 percent ownership of Sack Grain Company on January 1, 20X4, for $118,800 when the fair value of Sack's net assets was $19,000 higher than its $113,000 book value. The...
-
Assets Cash Prepaid Expenses Trade Receivables Inventories Property, Plant 2 CompuTech Income Statement 3 December 31, 2021 4 Sales Revenue S 360,000 3 6 Cost of Goods Sold 7 RM Purchases: $ 132,000...

Study smarter with the SolutionInn App


