Question: For the optimum temperature progression in a plug flow reactor in Example 9.4 (C A0 = 4 mol/liter, F A0 = 1000 mol A/min, X
For the optimum temperature progression in a plug flow reactor in Example 9.4 (CA0 = 4 mol/liter, FA0 = 1000 mol A/min, XA0 = 0.8, T = 5°C, Tmax = 95°C) and feed and product both at 25°C, how much heating and cooling would be needed.
(a) For the feed stream?
(b) In the reactor itself?
(c) For the stream leaving the reactor?
Example 9.4
Using the optimal temperature progression in a plug flow reactor for the reaction
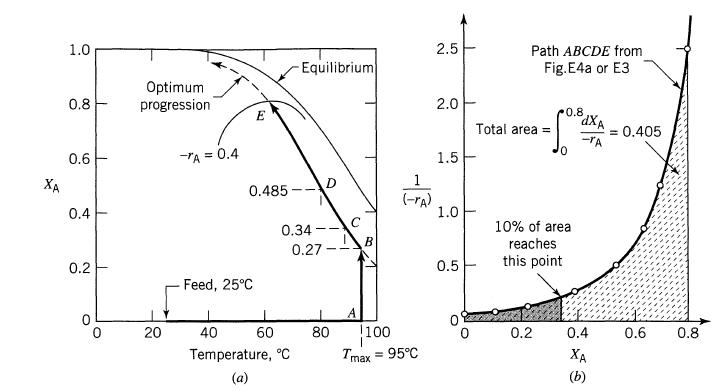
XA 1.0 0.8 0.6 0.4 0.2 1 Optimum progression 20 -TA = 0.4 E 0.485 Feed, 25C Equilibrium 0.34- 0.27 40 60 Temperature, C 0 80 C A B (-rA) 1100 I Tmax = 95C 2.5 2.0 1.5 1.0 0.5 0 Path ABCDE from Fig. E4a or E3 Total area = 0.8 0.2 0 10% of area reaches this point dXA -TA 0.4 XA = 0.405 0.6 0.8
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
a To calculate the heating required for the feed stream we need to know the heat capacity of the feed and the temperature change required The heat capacity of the feed can be calculated using the foll... View full answer

Get step-by-step solutions from verified subject matter experts


