Question: In slurry reactors, pure reactant gas is bubbled through liquid containing suspended catalyst particles. Let us view these kinetics in terms of the film theory,
In slurry reactors, pure reactant gas is bubbled through liquid containing suspended catalyst particles. Let us view these kinetics in terms of the film theory, as shown in Fig. P17.3. Thus, to reach the surface of the solid, the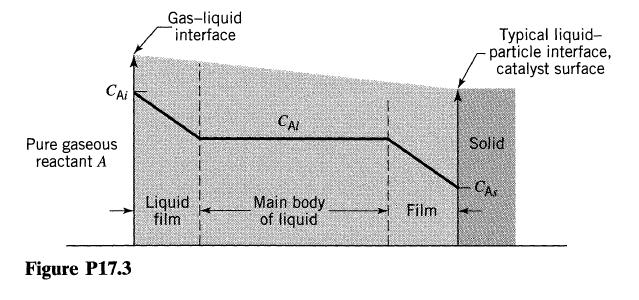
reactant which enters the liquid must diffuse through the liquid film into the main body of liquid, and then through the film surrounding the catalyst particle. At the surface of the particle, reactant yields product according to first-order kinetics. Derive an expression for the rate of reaction in terms of these resistances.
CAI Pure gaseous reactant A Figure P17.3 Gas-liquid interface Liquid film CAL Main body of liquid Film Typical liquid- particle interface, catalyst surface Solid CAS
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
The rate of reaction is given by the following formula To derive the expression for t... View full answer

Get step-by-step solutions from verified subject matter experts


