Orthonitroanaline (an important intermediate in dyescalled fast orange) is formed from the reaction of orthonitrochlorobenzene (ONCB) and
Question:
Orthonitroanaline (an important intermediate in dyes—called fast orange) is formed from the reaction of orthonitrochlorobenzene (ONCB) and aqueous ammonia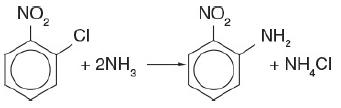
Orthonitrochlorobenzene and two molecules of ammonia (2NH3) react to form orthonitroaniline and ammonium chloride (NH4Cl).
The liquid-phase reaction is first order in both ONCB and ammonia with k = 0.0017 m3/kmol · min at 188°C with E = 11273 cal/mol. The initial entering concentrations of ONCB and ammonia are 1.8 and 6.6 kmol/m3, respectively.
a. Set up a stoichiometric table for this reaction for a flow system.
b. Write the rate law for the rate of disappearance of ONCB in terms of concentration.
c. Explain how parts (a) and (b) would be different for a batch system.
d.Write –rA solely as a function of conversion. –rA = ______
e. What is the initial rate of reaction (X = 0)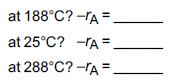
f. What is the rate of reaction when X = 0.90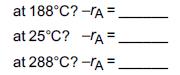
g. What would be the corresponding CSTR reactor volume at 25°C to achieve 90% conversion and at 288°C for a feed rate of 2 dm3/min
Step by Step Answer:






