The irreversible reaction A B is taking place in the same porous catalyst slab shown in
Question:
The irreversible reaction A → B is taking place in the same porous catalyst slab shown in Figure P15-9A.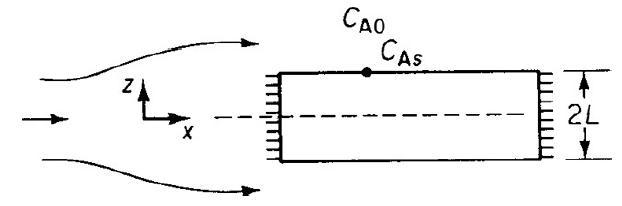
The reaction is zero order in A.
a. Show that the concentration profile using the symmetry B.C. is
CACAs=1+ϕ02[(zL)2−1](P15-10.1)
where
ϕ02=kL22DeCAs(P15-10.2)
b. For a Thiele modulus of 1.0, at what point in the slab is the concentration zero? For ϕ0 = 4?
c. What is the concentration you calculate at z = 0.1 L and ϕ0 = 10.
d. Plot the dimensionless concentration profile ψ = CA/CAs as a function of λ = z/L for ϕ0 = 0.5, 1, 5 and 10. Hint: there are regions where the concentration is zero. Show that λC = (1 – 1/ϕ0) is the start of this region where the gradient and concentration are both zero. (L. K. Jang, R. L. York, J. Chin, and L. R. Hile, Inst. Chem.
Engr., 34, 319 (2003).)
Show that ψ=ϕ02λ2−2ϕ0(ϕ0−1)λ+(ϕ0−1)2 for λC≤λe. The effectiveness factor can be written as
f. Make a sketch for η versus ϕ0 similar to the one shown in Figure 15-5.
g. Repeat parts (a) through (f) for a spherical catalyst pellet.
Figure 15-5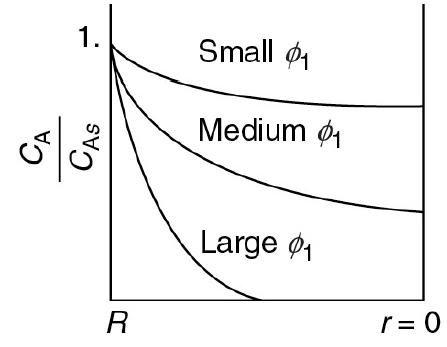
Step by Step Answer:






