Under ultraviolet radiation, reactant A of C A0 = 10 kmol/m 3 in a process stream (v
Question:
Under ultraviolet radiation, reactant A of CA0 = 10 kmol/m3 in a process stream (v = 1m3/min) decomposes as follows.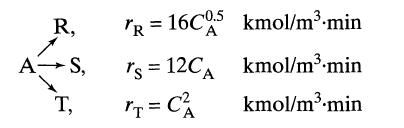
We wish to design a reactor setup for a specific duty. Sketch the scheme selected, and calculate the fraction of feed transformed into desired product as well as the volume of reactor needed.
Product R is the desired material.
Transcribed Image Text:
R, A-S, T, TR = 16C0.5 kmol/m³.min A rs 12CA = T = C² kmol/m³.min kmol/m³.min
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
How should we operate a mixed flow reactor so as to maximize the production of R? Separation and recycle of unused reactant is not practical. Under ultraviolet radiation, reactant A of C A0 = 10...
-
Under ultraviolet radiation, reactant A of C A0 = 10 kmol/m 3 in a process stream (v = 1m 3 /min) decomposes as follows. We wish to design a reactor setup for a specific duty. Sketch the scheme...
-
Under ultraviolet radiation, reactant A of C A0 = 10 kmol/m 3 in a process stream (v = 1m 3 /min) decomposes as follows. We wish to design a reactor setup for a specific duty. Sketch the scheme...
-
Let Z[] be the following set of rational numbers { a Z, k N} (and recall that N = {0, 1, 2,...} in this class). Let the addition and multiplication for Z[] be the usual addition and multiplication...
-
A mutual fund charges a 5% upfront load plus reports an expense ratio of 1.34%. If an investor plans on holding a fund for 30 years, what is the average annual fee, as a percent, paid by the...
-
You have the chance to participate in a project that produces the following cash flows: 4,400 Cash Flows ($) C 4,600 -10,600 a. The internal rate of return is 11.51%. If the opportunity cost of...
-
The U.S. economy was in recession during part of 1991, most of 2001, and from December 2007 through June 2009. Compute the residuals for the least-squares line found in Exercise
-
Scrap at time of sale or at time of production, journal entries (continuation of 18-35). Assume that Job #10 of Crystal Clear Machine Shop generates normal scrap with a total sales value of $650 (it...
-
You are presented with the following transactions for Paddick Enterprises Ltd. for the month of February: Feb 2 purchased supplies on account, $600. 3 Purchased equipment for $10,000 by signing a...
-
The stoichiometry of a liquid-phase decomposition is known to be In a series of steady-state flow experiments (C A0 = 100, C RO = C SO = 0) in a laboratory mixed flow reactor the following results...
-
Substance A in a liquid reacts to produce R and S as follows: A feed (C A0 = 1, C R0 = 0, C S0 = 0) enters two mixed flow reactors in series, ( 1 = 2.5 min, 2 = 5 min). Knowing the composition in...
-
The Second Derivative Test for Local Maxima and Minima says: a. has a local maximum value at x = c if (c) = 0 and (c) b. has a local minimum value at x = c if (c) = 0 and (c) > 0. To prove...
-
On January 1, 20X1, Elberta Company issued $50,000 of 4% convertible bonds, in total, into 5,000 shares of Elberta's common stock. No bonds were converted during 20X1. Throughout 20X1 Elberta had...
-
At Vision Club Company, office workers are employed for a 40-hour workweek and are quoted either a monthly or an annual salary (as indicated). Given on the form below are the current annual and...
-
8 Project two 15 UTSA Project two M Question 1 - Project two ChatGPT C chegg.com/homework-he X Course Hero how to take a sxreen shot X +...
-
Charitable purposes: Section 3(1) Charities Act 2011 1. Prevention or relief of poverty 2. Education 3. Religion, now includes: 4. - - A religion which involves belief in more than one god A religion...
-
Jack Price, The finance director of Humpty Doo Investment Ltd ( HDIL ) , is unsure whether he should consolidate some of the investments that the company owns. He has asked your advice as business...
-
When Mary Potts arrived at her store on the morning of January 29, she found empty shelves and display racks; thieves had broken in during the night and stolen the entire inventory. Accounting...
-
In Exercises delete part of the domain so that the function that remains is one-to-one. Find the inverse function of the remaining function and give the domain of the inverse function. f(x) = 16x4 -3...
-
The reactions of ozone were studied in the presence of alkenes (from R. Atkinson et al., Int. J. Chem. Kinet., 15(8), 721). The data in Table P7-10C are for one of the alkenes studied, cis-2-butene....
-
Tests were run on a small experimental reactor used for decomposing nitrogen oxides in an automobile exhaust stream. In one series of tests, a nitrogen stream containing various concentrations of NO...
-
The thermal decomposition of isopropyl isocyanate was studied in a differential packed-bed reactor. From the data in Table P7-12A, determine the reactionrate-law parameters. TABLE P7-12A RAW DATA Run...
-
For each scenario apply the six-step rpocedure for dealing with conflict. Your Task Analyze the following scenarios. In teams, discuss each scenario and apply the six-step procedure for dealing with...
-
Which of the following is true? Lower-level managers are responsible for preparing the entire budget. The budget and the administration of the budget are the responsibility of management. The flow of...
-
Instructions The following items were selected from among the transactions completed by Sherwood Co. during the current year: Mar. 1 Purchased merchandise on account from Kirkwood Co., $390,000,...

Study smarter with the SolutionInn App


