What if... you were asked to rework Example 1-2 to calculate the time to reduce the number
Question:
What if... you were asked to rework Example 1-2 to calculate the time to reduce the number of moles of A to 1% if its initial value for a constant volume BR, what would you say? Would you do it? If your answer is “yes,” go ahead and calculate it; if your answer is “NO, I won’t do it!” then suggest two ways to work this problem incorrectly.
Transcribed Image Text:
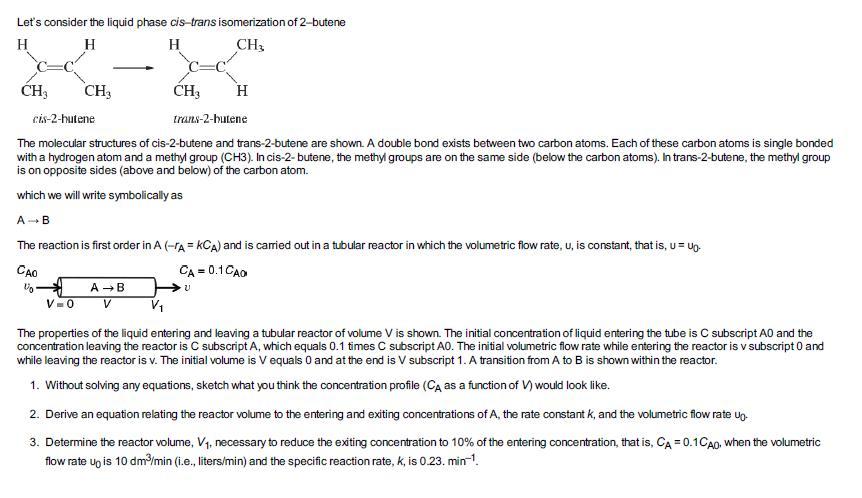
Let's consider the liquid phase cis-trans isomerization of 2-butene H H H CH3 CH3 CH3 V=0 CH3 cis-2-hutene trans-2-butene The molecular structures of cis-2-butene and trans-2-butene are shown. A double bond exists between two carbon atoms. Each of these carbon atoms is single bonded with a hydrogen atom and a methyl group (CH3). In cis-2-butene, the methyl groups are on the same side (below the carbon atoms). In trans-2-butene, the methyl group is on opposite sides (above and below) of the carbon atom. which we will write symbolically as A B The reaction is first order in A (-A = KCA) and is carried out in a tubular reactor in which the volumetric flow rate, u, is constant, that is, u = Up- CAO CA=0.1 CAO U₂ A → B V H V The properties of the liquid entering and leaving a tubular reactor of volume V is shown. The initial concentration of liquid entering the tube is C subscript AO and the concentration leaving the reactor is C subscript A, which equals 0.1 times C subscript A0. The initial volumetric flow rate while entering the reactor is v subscript 0 and while leaving the reactor is v. The initial volume is V equals 0 and at the end is V subscript 1. A transition from A to B is shown within the reactor. 1. Without solving any equations, sketch what you think the concentration profile (CA as a function of V) would look like. 2. Derive an equation relating the reactor volume to the entering and exiting concentrations of A, the rate constant k, and the volumetric flow rate up- 3. Determine the reactor volume, V₁, necessary to reduce the exiting concentration to 10% of the entering concentration, that is, CA = 0.1CA0, when the volumetric flow rate up is 10 dm³/min (i.e., liters/min) and the specific reaction rate, k, is 0.23. min-¹.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
Based on the image provided you are dealing with a firstorder reaction for the isomerization of cis2...View the full answer

Answered By

Joan Gakii
I'm a meticulous professional writer with over five years writing experience. My skill set includes
- Digital Content,
- Interpersonal Communication,
- Web Content and academic Writing,
- Proofreading,
- Editing,
- Project Management, and
- Public Relations.
5.00+
7+ Reviews
12+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
This short paper sets up the assumptions portion of your research that will be used in paper 3. The focus is on "all else constant." The two states you will consider for both this assignment and...
-
What follows is part of the testimony from Troy Normand in the WorldCom case. He was a manager in the corporate reporting department and is one of five individuals who pleaded guilty. He testified in...
-
The following is abbreviated testimony from Troy Normand in the WorldCom (USA) case. He was a manager in the company reporting department and was one of five individuals who pleaded guilty. He...
-
Find the minimum and maximum values of (x, y) = x 2 y on the ellipse 4x 2 + 9y 2 = 36.
-
Equipment acquired on January 3, 2004, at a cost of $96,000, has an estimated useful life of six years and an estimated residual value of $6,000. a. What was the annual amount of depreciation for the...
-
The Yummy Ice Cream Company projects the demand for ice cream by using exponential smoothing. Last week the forecast was 100,000 gallons of ice cream, and 90,000 gallons was actually sold. a. Using ...
-
Spring break is a popular travel occasion for many college students. What would be the motivation factors of spring break travel? Can you classify the motivation factors based on one of the tourist...
-
As a Netflix member, youre pleasantly surprised to find that the 1940s serial Zorros Black Whip has shown up on your recommended list. You move it to the top of your queue immediately, but youre...
-
2 Based on your analysis, when will the product category penetrate 50% of the market? O Sometime in the year 2028 O Sometime in the year 2029 By the end of the year 2029 4 pts None of the above
-
Will or could there ever be a case where the CSTR volume is represented by something other than a square or rectangle on a Levenspiel plot?
-
Water in an open (source or supply) tank is pumped to a second (destination) tank at a rate of 5 lb/sec with the water level in the destination tank 25 ft above the water level in the source tank,...
-
In Problems 6374, perform the indicated operations and simplify the result. Leave your answer in factored form. 3x x - 1 x - 4 18 x - 2x + 1 2
-
TranscribedText: El. You are sitting at a table that has a solid round top {5.1"} kg] and a single solid cvlindrical leg {4. kg) [see figure, note that the tilt angle is exaggerated to he...
-
The municipal mill rate in the neighbourhood is 22.375 mills. There is an educational mill rate of 11.35 mills. The following list is the municipalities planned local improvement costs for the next...
-
Maggie Company had the following functional income statement for the month of May, 2020: MAGGIE COMPANY Functional Income Statement For the Month Ending May 31, 2020 Sales (30,000 units) $300,000...
-
Leslie Sporting Goods is a locally owned store that specializes in printing team jerseys. The majority of its business comes from orders for various local teams and organizations. While Leslie's...
-
Euclid acquires a 7-year class asset on May 9, 2022, for $153,000 (the only asset acquired during the year). Euclid does not elect immediate expensing under 179. He does not claim any available...
-
Commuters In Problems 1-2, find all the 2 x that commute with the given matrix. 2 matrices. 1. Where a ( R 2. Where k ( R, k ( 0 3 0 0 0 0
-
Identify the Critical Infrastructure Physical Protection System Plan.
-
Why is the buoyancy correction equal to 1 in Figure 2-9 when the density of the object being weighed is 8.0 g/mL? Figure 2-9
-
Pentane (C 5 H 12 ) is a liquid with a density of 0.626 g/mL near 25C. Find the true mass of pentane when the mass in air is 14.82 g. Assume air density = 0.001 2 g/mL.
-
The densities (g/mL) of several substances are: acetic acid, 1.05; CCl 4 , 1.59; S, 2.07; Li, 0.53; Hg, 13.5; PbO 2 , 9.4; Pb, 11.4; Ir, 22.5. From Figure 2-9, predict which substances will have the...
-
Textbook Problem Materials 6-23 Investments-Cash Dividends. D, Inc. had accumulated earnings and profits at January 1 of the current year of $20,000. During the taxable year, it had current earnings...
-
Which of the following statements is correct? Select one: a. If the same people are responsible for a series of related accounting activities is called Segregation of Duties. b. Auto generated...
-
answer B, incorrect answer is 13.04. cant figure it out 15/30 Question 2 of 3 CAT Westermarchever change forwart The cocodrig de coming prach for setting it. The comproduction the battery.com de for...

Study smarter with the SolutionInn App


