A 1.00-L mixture of helium, neon, and argon has a total pressure of 662 mmHg at 298
Question:
A 1.00-L mixture of helium, neon, and argon has a total pressure of 662 mmHg at 298 K. If the partial pressure of helium is 341 mmHg and the partial pressure of neon is 112 mmHg, what mass of argon is present in the mixture?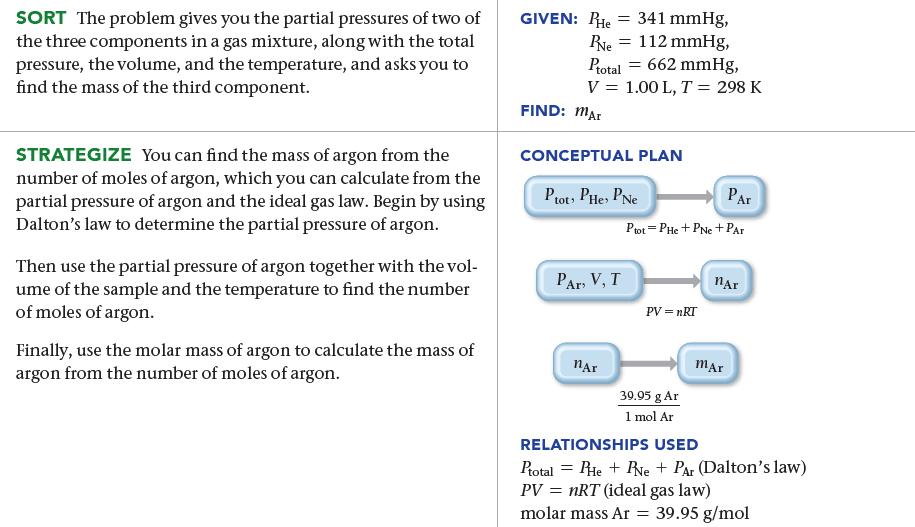
Transcribed Image Text:
SORT The problem gives you the partial pressures of two of the three components in a gas mixture, along with the total pressure, the volume, and the temperature, and asks you to find the mass of the third component. STRATEGIZE You can find the mass of argon from the number of moles of argon, which you can calculate from the partial pressure of argon and the ideal gas law. Begin by using Dalton's law to determine the partial pressure of argon. Then use the partial pressure of argon together with the vol- ume of the sample and the temperature to find the number of moles of argon. Finally, use the molar mass of argon to calculate the mass of argon from the number of moles of argon. GIVEN: PHE = 341 mmHg, Re = 112 mmHg, Ptotal = 662 mmHg, V = 1.00 L, T = 298 K FIND: MAT CONCEPTUAL PLAN Plot PHe, PNe PAT, V, T nAr PAR Ptot - PHe + PNE + PAT PV = nRT 39.95 g Ar 1 mol Ar nAr mar RELATIONSHIPS USED Ptotal = He + Ne + Par (Dalton's law) PV = nRT (ideal gas law) molar mass Ar = 39.95 g/mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Ptotal PHe RNe PAT PAr Ptotal PH PNe 662 mmHg 341 mmHg 1...View the full answer

Answered By

BETHUEL RUTTO
Hi! I am a Journalism and Mass Communication graduate; I have written many academic essays, including argumentative essays, research papers, and literary analysis. I have also proofread and written reviews, summaries and analyses on already finished works. I am eager to continue writing!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A 1.50-L mixture of helium, neon, and argon has a total pressure of 754 mmHg at 310 K. If the partial pressure of helium is 431 mmHg and partial pressure of neon is 211 mmHg, what mass of argon is...
-
1 723 Conditions for promotions 5 Years of service (Years) 6 Psychometric test (%) Required: a) LIST OF EMPLOYEES FOR PROMOTION 9 Names of employees Years of service 10 Munawarah Ali 11 Amiruddin...
-
Consider the reaction 2 NO2(g) N2O4(g). (a) Using data from Appendix C, calculate G at 298 K. (b) Calculate G at 298 K if the partial pressures of NO2 and N2O4 are 0.40 atm and 1.60 atm,...
-
Explain the nature of stress at work Describe the health consequences of stressful work Explain how to use hardiness theory to reduce stress List three ways to use Banduras self-efficacy theory to...
-
What are blogs? How are they used? Who is using them?
-
Considera2-year,risk-freebondwithacouponrateof6%(annualcoupons)andafaceamount of$1,000. a.What ispriceofthisbondiftheYTMis5%?6%?7%?...
-
Identify those organizational structures that result in paying income taxes based on distributed, as compared to earned, profits. Explain the advantages of each approach. AppendixLO1
-
The following account balances were selected from the records of TEAC Corporation at December 31, 2011, after all adjusting entries were completed: Common stock (par $20; authorized 100,000 shares,...
-
Oracle Corporation, a US firm, just signed a contract to provide computer services to Fujitsu of Japan over a 10-year period and receive payments in yen. To manage the operating exposure, Oracle has...
-
Activity-based budgeting, Balanced Scorecard, and strategy Sippican Corporation (B)12 Refer to Case 5-36, the Sippican Corporation (A) case, which required time-driven ABC analysis. Sippican's senior...
-
Why is it impossible to breathe air through an extra-long snorkel (longer than a couple of meters) while swimming under water?
-
A gas sample at STP contains 1.15 g oxygen and 1.55 g nitrogen. What is the volume of the gas sample? a) 1.26 L b) 2.04 L c) 4.08 L d) 61.0 L
-
Revisit the copying service in Example 8.1 and assume that over the years, the monthly demand from the four customers has increased to the following weights: w 1 = 7, w 2 = 9, w 3 = 5, w 4 = 7. If we...
-
According to a recent study, 21% of American college students graduate with no student loan debt. Suppose we obtain a random sample of 106 American college students and record whether or not they...
-
Differentiate the following with respect to x: a. y=5x+2x + x + 15 b. y=4x+3x - 4x - 10 c. y = 3Sin(5x) d. y = 3Cos(3x) e. y=10e -25x f. y = log(6x)
-
Question 2. The rate of drug destruction by the kidneys is proportional to the amount of the drug in the body. The constant of proportionality is denoted by K. At time t the quantity of the drug in...
-
5. 6. -1 (4a) U u X2 1 X2 -2 x -1 -2 12 (4b) U -2 2 Y y 16 x2 X2 3 1 (4c) U u - x 2 Y y -8 Y y -20 5 x X2 2 Find the state space models of the three systems shown in Fig. 4a, Fig. 4b, and Fig. 4c,...
-
Given the following data for Mehring Company, compute total manufacturing costs, prepare a cost of goods manufactured statement, and compute cost of goods sold. Direct materials used $230,000...
-
Using the isothermal transformation diagram for a 0.45 wt% C steel alloy (Figure 10.39), determine the final microstructure (in terms of just the micro constituents present) of a small specimen that...
-
r = 0.18 Find the coefficients of determination and non-determination and explain the meaning of each.
-
Determine the moments at B and C, then draw the moment diagram for the beam. Assume the supports at B and C are rollers and A and D are pins. EI is constant. 12 kN/m 12 kN/m A 4 m 6 m 4 m
-
Determine the reactions at the supports. Assume A is fixed and B and C are rollers that can either push or pull on the beam. EI is constant. 12 kN/m B. -2.5 m 5 m
-
Determine the moments at B and C, then draw the moment diagram for the beam. All connections are pins. Assume the horizontal reactions are zero. EI is constant. 12 kN/m 4 m- 12 kN/m D. -4 m 4 m-
-
Winter Time Adventures is going to annual dividend if $2.61 a share on its common stock next week. This year, the company paid a dividend of $2.50 a share. The company adheres to a constant rate of...
-
Small Factory : -Regular time 8 hours per day. -1 hour daily lunch break. -25 working days per month. -50 workers. -Worker productivity 2.5 units per hour. -sold for $ 150 per unit. -cost of Labor...
-
$500 is invested for 7 years at 10 % p.a. simple interest. How much will the investment be worth after this period

Study smarter with the SolutionInn App


